Cleavage refers to the stereotyped pattern of early mitotic divisions that divides up the large volume egg cytoplasm. The early zygote is unique in being so large. Most cells undergo a period of growth between cycles of mitosis, but this is not true for early cleavage stage blastomeres. With each division the cells get smaller. This rapid pattern of cell division without concomitant growth abruptly halts at the stage called the mid-blastula transition where the zygotic nucleus takes control of the cell cycle.
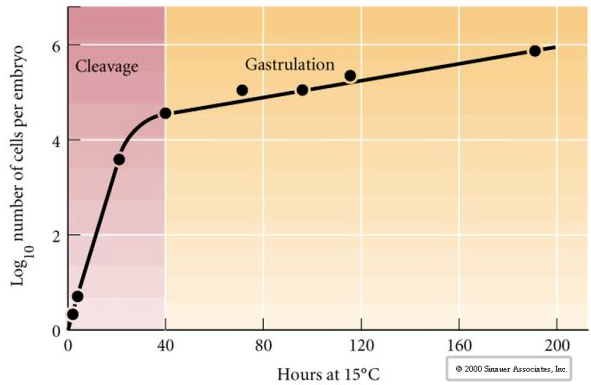 There is some evidence that a maternal factor, perhaps a transcriptional regulator, is responsible for this early rapid pattern of cleavage divisions. By artificially altering the cytoplasmic to nuclear DNA ratio you can change the time of the midblastula transition. The midblastula transition refers to the time when the major switch from expression of maternal to zygotic genes takes place.
There is some evidence that a maternal factor, perhaps a transcriptional regulator, is responsible for this early rapid pattern of cleavage divisions. By artificially altering the cytoplasmic to nuclear DNA ratio you can change the time of the midblastula transition. The midblastula transition refers to the time when the major switch from expression of maternal to zygotic genes takes place.
Fertilization in some species leads to radical cytoplasmic movements that are essential for ensuring the cytoplasmic determinants are located in the correct positions relative to subsequent cleavage events.
PATTERNS OF EMBRYONIC CLEAVAGE
Pattern of embryonic cleavage is determined both by the position of the mitotic spindles and by the amount and distribution of yolk. Yolk tends to inhibit cleavage. It slows it down or actually prevents complete cleavage. Yolk is an adaptation of those animals that go through more or less of embryogenesis isolated from any food supply. Some animals, like sea urchin, have relatively little yolk because they rapidly develop into a free swimming larval form that acquires nutrients from their environment. Other animals such as marsupials are born prematurely, but are provided nourishment in a parental pouch. Placental mammals develop a specialized organ through which the embryo is nourished throughout development and so also have little yolk.
The types of eggs based on yolk characteristics are described as:
Isolecithal: sparse evenly distributed yolk, eg., sea urchin, mouse
Mesolecthal: moderate amount of yolk, often unevenly distributed, eg., frog
Telolecithal: dense yolk concentrated at one end, eg., bird, reptile
Centrolecithal: yolk concentrated at the middle of the egg, eg. fly
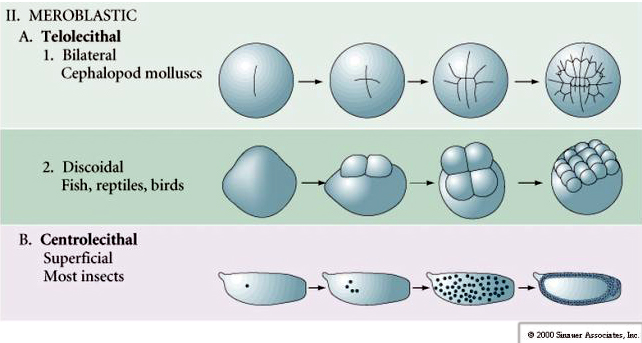
Many eggs are polarized with a yolk rich pole, termed the vegetal pole and a yolk poor pole termed the animal pole, eg., frog. The zygotic nucleus is generally displaced towards the animal pole. Zygotes with relatively little yolk (isolecithal and mesolecithal) cleave HOLOBLASTICALLY. The cleavage furrow extends all the way through the egg. While telolecithal and centrolecithal zygotes undergo MEROBLASTIC cleavage where the cleavage plane extends only to the accumulated yolk. In centrolecithal eggs (many insect eggs) cleavage is meroblastic and superficial, while in telolecithal eggs (birds and fish) cleavage is discoidal
There are several types of cleavage symmetry seen in nature: radial(echinoderms, amphibians), spiral (mollusks, annelids), Bilateral (ascidians,tunicates), Rotational (mammals). The two figures below show examples of holoblastic and meroblastic cleavage symmetries.
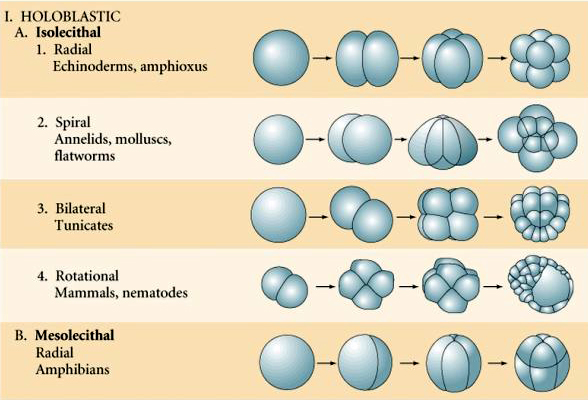
RADIAL HOLOBLASTIC CLEAVAGE
Excellent movie of sea urchin cleavage from Rachel Fink's "A Dozen Eggs".
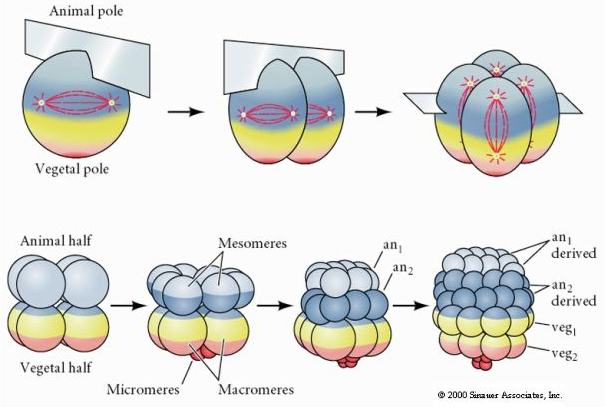
Sea urchins also have radial holoblastic cleavage, but with some interesting differences. First cleavage is meridional.Second cleavage is meridional. Third cleavage is equatorial Fourth cleavage is meridional, but while the four animal pole cells split equally to give rise to eight equal sized animal blastomeres termed MESOMERES, the vegetal cells divide asymmetrically along the equatorial plane to give 4 large MACROMERES and 4 much smaller MICROMERES at the vegetal pole. Fifth division the MESOMERES divide equatorially to give two tiers of eight MESOMERES an1 and an2 , the MACROMERES divide meridionally forming a tier of eight cells below an2, the MICROMERES divide to give a cluster of cells below the veg1 layer. The sixth divisions are all equatorial, giving a veg2 layer. The seventh divisions are all meridional giving a 128 cell blastula.
What determines these cleavage patterns? Are they dependent on the previous cleavage and played out like a tape or are they determined by some intrinsic clock? In 1939 Horstadius inhibited one or two of the first three cleavages and found the appearance of the micromeres occurred at the right time regardless of the history of cleavages
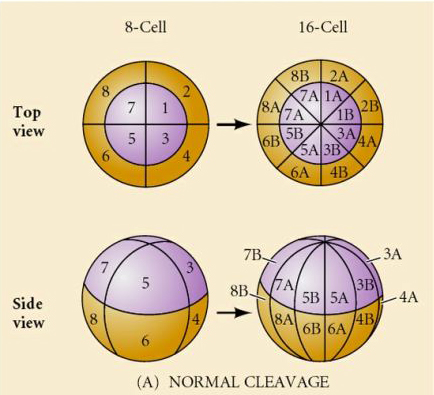
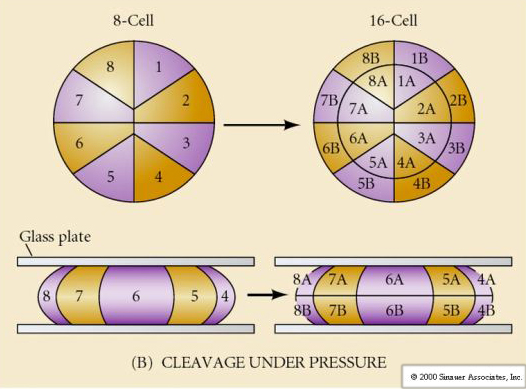
The conclusion from these experiments is that there is some factor in the vegetal pole of the egg that determines the formation of the micromeres and further that there must be a “molecular clock” that starts at egg activation. The clock is independent of the actual cleavage event.
The 128 cell blastula is a rather loose ball of cells surrounding a hollow blastocoel. The ball is one cell layer thick with all cells in contact with the external hyaline layer and the internal fluid of the blastocoel. At this stage in development the cells begin to form the tight junctions characteristic of an epithelium. The central blastocoel is now isolated from the external environment. The blastomeres continue to divide with their axis parallel to the hyaline layer, remaining a epithelium one cell thick. The blastocoel continues to enlarge.
Two theories attempt to account for the pattern of enlargement of the blastocyst
1. The osmotic theory suggests that ions and proteins are secreted into the blastocoel by the blastomeres and this results in a pressure buildup due to the osmotic flow of water. This pressure would then be responsible for aligning the axis mitosis of the blastomeres and the enlargement of the blastocoel.
2. The alternate theory by Wolpert and his colleagues suggests that it is really the adhesive interactions among the blastomeres and between the blastomeres and the hyaline layer that aligns the mitotic axis's. That is the adhesion to the hyaline is greatest, the adhesion to other blastomeres is next, and finally the interaction with the blastocoel wall is least. The dominant adhesion with the hyaline layer forces the expansion of the blastocyst and blastocoel.
The cells of the blastula grow cilia on their outer surface, secrete a “hatching enzyme” (hyalinase) and become free swimming.
AMPHIBIAN CLEAVAGE
Cleavage in many amphibians is holoblastic with radial symmetry, however the large volume of yolk (its mesolecithal) interferes with cleavage. At the animal pole first cleavage proceeds at about 1mm/min, while through the vegetal pole is proceeds 50-100 times slower (.02mm/min). While the first cleavage is still incomplete in the yolky vegetal region of the egg the second meridional cleavage begins to take place.
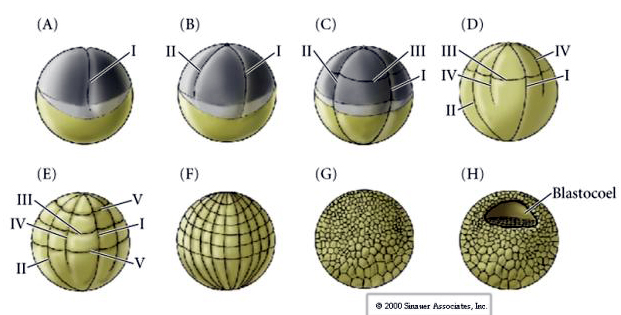
The third cleavage is equatorial, but because the nuclei and asters are displaced “animal-ward” the cleavage plane although perpendicular to the animal vegetal axis is also displaced towards the animal pole and does not equally divide the blastomeres. The result is four smaller animal blastomeres (termed MICROMERES) and four large vegetal pole blastomeres (termed MACROMERES). This unequal holoblastic cleavage gives rise to a more rapidly dividing animal pole made up of smaller micromeres and a slower dividing vegetal pole made up of macromeres. The animal pole soon is composed of many small micromeres and the vegetal pole a few yolk filled large macromeres. Although the formation of the blastocoel begins with the first cleavage, it does not become obvious until the 128 cell stage.
WHAT FUNCTION DOES THE BLASTOCOEL SERVE?
The blastocel spatially separates cells so they do not touch one another. Cells at the roof of the blastocoel normally become ectoderm. If you transplant cells from the roof of the blastocoel next to the yolky cells at the base of the blastocoel they will differentiate as mesoderm. Mesodermal derivatives are normally produced from cells adjacent to the endodermal precursors. One possibility that we will thoroughly explore is that the vegetal cells “induce” via cell-cell interactions the adjacent cells to become mesodermal. Thus the formation of the blastocoel may be necessary to prevent inappropriate "inductive" interactions among early cells of the blastocyst. The second obvious need for the blastocoel may be during the subsequent stage of development, GASTRULATION, where cells migrate into the interior of the blastocoel.
MAMMALIAN CLEAVAGE
The mammalian egg is released from the ovary into the oviduct where it is fertilized. First cleavage begins about a day after fertilization within the oviduct. In sharp contrast to most animals, cleavage in mammals can be very slow---1/day.
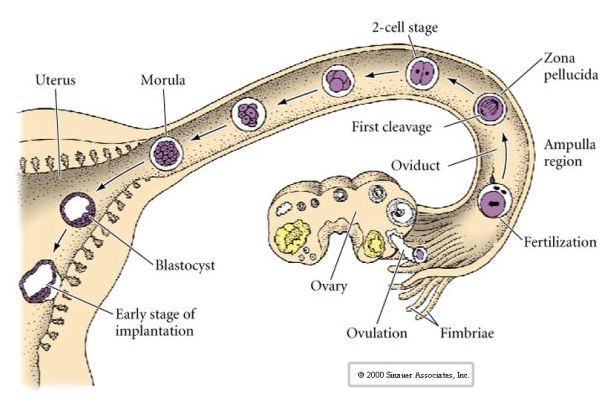
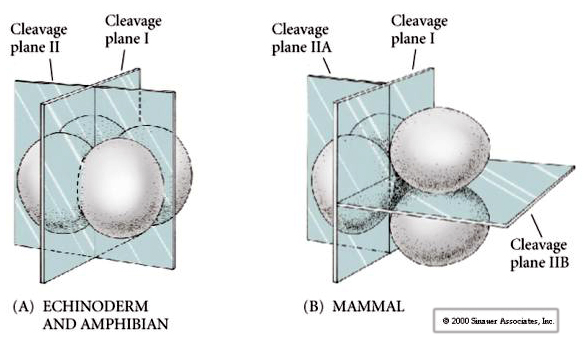 Additionally, the cleavage planes are somewhat different from other animals. First cleavage is meridional just like sea urchin and frog. However, the second cleavage division sees one of the blastomeres dividing meridionally and the other equatorially! This type of cleavage is called ROTATIONAL HOLOBLASTIC CLEAVAGE.
Additionally, the cleavage planes are somewhat different from other animals. First cleavage is meridional just like sea urchin and frog. However, the second cleavage division sees one of the blastomeres dividing meridionally and the other equatorially! This type of cleavage is called ROTATIONAL HOLOBLASTIC CLEAVAGE.
Another unique feature of mammalian cleavage is that the blastomere cleavages are asynchronous. (compared with the synchrony of sea urchin and frog up till the midblastula transition). Cleavage of the mammalian embryo is regulated by the zyotic nucleus from the very start.
Through the third cleavage the blastomeres form a ball of loosely associated cells just like the other animals we’ve studied. Before the fourth cleavage the cells of the blastula dramatically change their behavior towards one another. They rapidly try to maximize their contacts with the other blastomeres and in doing cause the blastula to compact.

This COMPACTION results in part from the production of an novel adhesion molecule UVOMORULIN (E-Cadherin) and is stabilized by the formation of tight junctions between the outer cells which like in the sea urchin seals off the interior of the blastula from the exterior. The cells also form gap junctions among themselves that allows the passage of small molecules, such as ions and some second messenger molecules such as Ca++ and C-AMP. The compacted 16 cell morula consists of an outer rind of cells and a few cells (1-2) completely internal. Most of the external cells give rise to the TROBLASTIC OR TROPHECTODERMAL CELLS. These cells do not contribute to the embryo proper, but instead are necessary for implantation of the embryo in the uterine wall and form the tissues of the CHORIAN, an essential component of the placenta that we’ll talk about later.
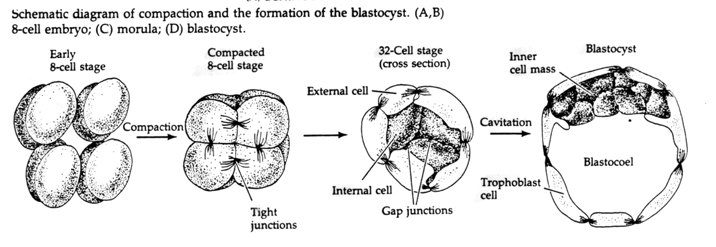
The cells of the embryo are derived from the inner few cells of the 16 cell stage blastula. These cells generate the inner cell mass of cell from which the entire embryo develops. By the 6th cleavage, the 64 cell stage the inner cell mass and trophoblastic layer are completely separate. The trophoblasts secret fluid internally to create the blastocoel. The embryo is now call a blastocyst.
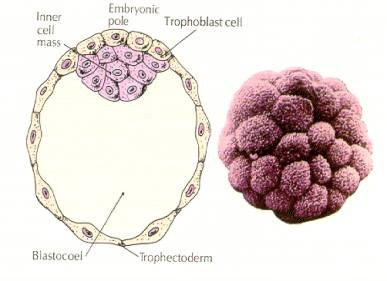 FORMATION OF THE INNER CELL MASS
FORMATION OF THE INNER CELL MASS
How are these inner cell mass cell created? Are there certain blastomeres fated by intrinsic factors to become inner cell mass progenitors? The answer seems to be no. All the early blastomeres seem to be totipotent and the determination of which cells will contribute to the trophoblastic layer and which to the inner cell mass simply a matter of chance position. Cells from a 4 cell stage embryo, which will normally give rise to both inner cell mass and trophectoderm cells, transplanted to the outside of a 32 cell stage embryo only give rise to trophectoderm. They do not contribute to the embryo proper. Remember from the earlier lecture on cloning that fusion of two 8 cell stage mouse embryos results in a normal embryo, suggesting that all the cells at that stage are totipotent.
MEROBLASTIC CLEAVAGE
In telolecithal and centrolecithal eggs the large dense yolk prevents cleavage. Telolecithal eggs are characteristic of birds, fishes, and reptiles while centrolecithal eggs are characteristic of insects. Telolecithal eggs result in meroblastic discoidal cleavage. Cleavage is restricted to the blastodisc at the animal pole of the egg. At early cleavages, because cleavage cannot proceed through the yolk, the blastomeres are continuous at their vegetal margins.
This movie of zebrafish development byRolf Karlstrom is excellent. (Movie by Paul Myers)
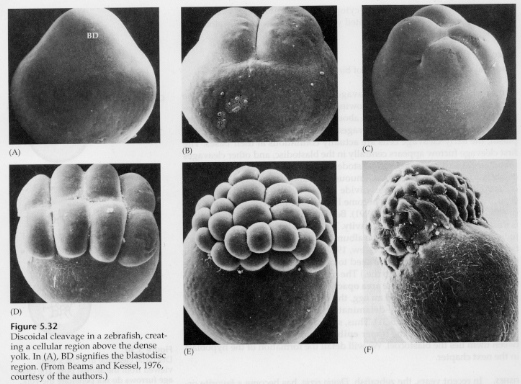 It's not until the equatorial cleavages that the cells of the blastoderm separate from the yolk. Further equatorial cleavages create a multilayered blastoderm three or four cells thick.
It's not until the equatorial cleavages that the cells of the blastoderm separate from the yolk. Further equatorial cleavages create a multilayered blastoderm three or four cells thick.
In birds a space forms between the blastoderm and the yolk called the SUBGERMINAL cavity. By the 16 division (60,000 cells) cells of the blastoderm migrate into the subgerminal cavity to form a second layer. The two layers are called the outer EPIBLAST and inner HYPOBLAST with the blastocoel between. We will study this in more detail latter when we discuss bird and mammal gastrulation
Centrolecital eggs of arthropods undergo a SUPERFICIAL CLEAVAGE. The large central mass of yolk confines the cleavages to the cytoplasmic rim of the egg.
An interesting and informative variation is seen in insects. The zygotic nuclei divide with out cleavage. That is the nuclei undergo karyokinesis----mitotic division of the nucleus--- without cytokinesis---the division of the cell. These naked nuclei are called ENERGIDS. The nuclei divide at an amazing rate---every 8 minutes (all of embryogenesis takes only 22 hrs).
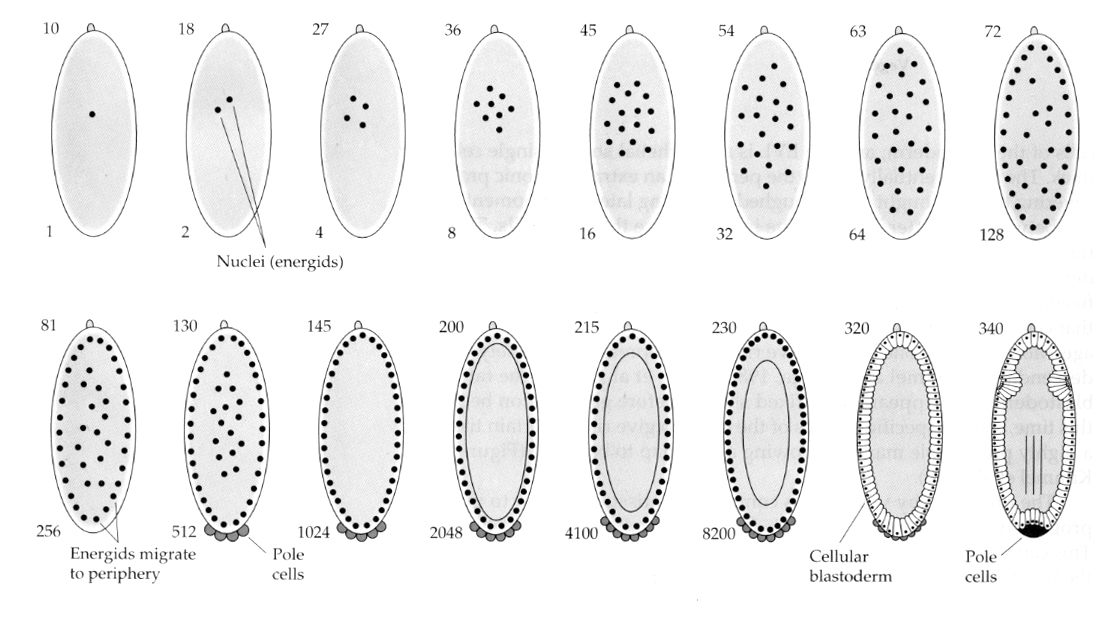
 After several rounds of karyokinesis the naked nuclei migrate to the periphery of the egg. At this stage it is called the SYNCYTIAL BLASTODERM because all the nuclei share the same cytoplasm. Cellularzation occurs at about the 14th nuclear division to create the CELLULAR BLASTODERM. After this time cells divide asynchronously. This corresponds to the midblastula transition of frogs and sea urchins. (transition from maternal to primarily zygotic gene expression) Remember that the midblastula transition was thought to be triggered by the ratio of chromatin to cytoplasm. Evidence for this mechanism in flies is seen by examining mutant haploid embryos. These embryos undergo the midblastula transition and cellularization one division later 15th. Furthermore you can accelerate cellularization by ligating the egg and reducing the volume of cytoplasm. Although the syncytial blastoderm stage suggests that all the nuclei are equipotent in that there do not seem to be diffusional barriers to cytoplasmic determinants, in fact the cytoplasm is very regionalized and the nuclei have highly organized cytoplasmic domains around them.
After several rounds of karyokinesis the naked nuclei migrate to the periphery of the egg. At this stage it is called the SYNCYTIAL BLASTODERM because all the nuclei share the same cytoplasm. Cellularzation occurs at about the 14th nuclear division to create the CELLULAR BLASTODERM. After this time cells divide asynchronously. This corresponds to the midblastula transition of frogs and sea urchins. (transition from maternal to primarily zygotic gene expression) Remember that the midblastula transition was thought to be triggered by the ratio of chromatin to cytoplasm. Evidence for this mechanism in flies is seen by examining mutant haploid embryos. These embryos undergo the midblastula transition and cellularization one division later 15th. Furthermore you can accelerate cellularization by ligating the egg and reducing the volume of cytoplasm. Although the syncytial blastoderm stage suggests that all the nuclei are equipotent in that there do not seem to be diffusional barriers to cytoplasmic determinants, in fact the cytoplasm is very regionalized and the nuclei have highly organized cytoplasmic domains around them.
MECHANISMS OF CLEAVAGE
Cell Cycle
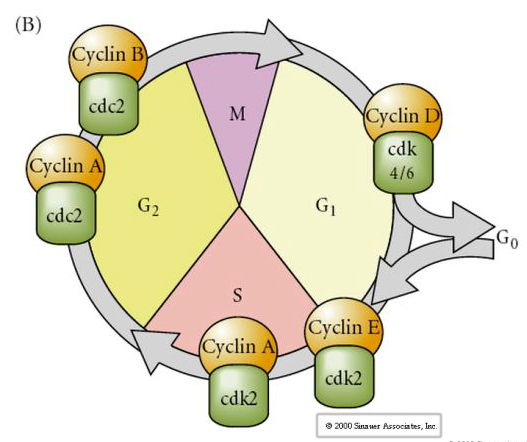
M-mitosis
G1- pre-replication gap
S- DNA synthesis
G2-premitotic gap
In cleavage stage embryos such as frogs and flies the blastomeres go directly from M to S without intervening G1 or G2 stages. After the midblastula transition cells in both animals have a G1 and G2. Elegant transplant experiments have demonstrated that it is the cytoplasm that regulates both karyokinesis and cytokinesis. If nuclei from dividing cells are transplanted into oocyte they immediately stop dividing.
 Conversely if nuclei from non-dividing cells are put into fertilized enucleated eggs they start dividing. Artificially activated enucleated eggs without centrioles will undergo cortical contractions reminiscent of cleavage. Some of the cytoplasmic factors regulating cell division in the early embryo have been identified.
Conversely if nuclei from non-dividing cells are put into fertilized enucleated eggs they start dividing. Artificially activated enucleated eggs without centrioles will undergo cortical contractions reminiscent of cleavage. Some of the cytoplasmic factors regulating cell division in the early embryo have been identified.
CYTOSTATIC FACTOR (CSF) is elevated after the first meiotic division and arrests the oocyte in the second meiotic metaphase. Upon fertilization the Ca inactivates CSF, meiosis is completed and the pronuclei fuse.
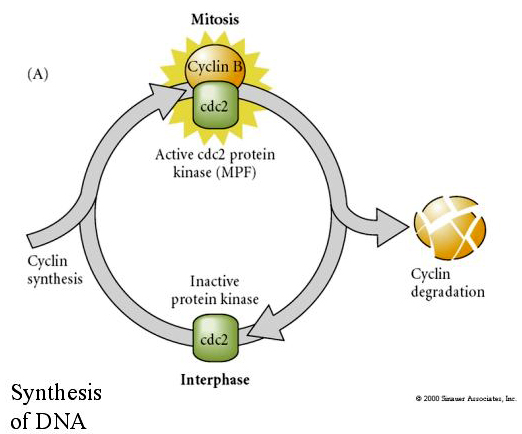
MITOSIS PROMOTING FACTOR (MPF) causes cells to enter M phase. MPF activation causes: 1. chromosome condensation by H1 histone phosphorylation, 2. nuclear envelope breakdown by hyperphosphorylation of 3 nuclear lamins, 3. RNA polymerase inhibition to shut down transcription, 4. Myosin regulatory subunit phosphorylation to inhibt cytokinesis.
Suggested model for cyclic regulation of cell cycle during cleavage stages of embrogenesis. MPF induces cell to proceed from S to M. CSF binds to MPF and prevents its inactivation. The cell remains in M. Ca increases and causes the inactivation of CSF which in turn leads to the inactivation of MPF and the cell proceeds through M to S and the cycle is repeated. MPF is made up of two subunits, Cyclin B and cdc2. It is cyclin B that undergoes a cell cycle specific synthesis and degradation regulated by the cells nucleus to control the cell cycle in normal somatic cells. However, during oogenesis the egg is loaded with "regulators" of cyclin B and cyclin B mRNA so that its syntheis is regulated by maternal factors independent of the zygotic nucleus. Thus it is not untill the maternal components "run out" that the zygotic nucleus takes over and a normal cell cycle (M, G1, S, G2) returns.
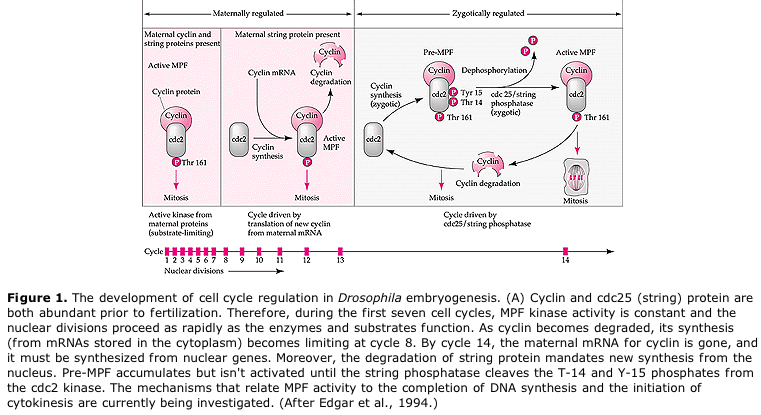
CELL FATE DETERMINATION
Cytoplasmic Localization of DETERMINANTS as a general and basic mechanism for early patterning (Examples Tunicate and Sea Urchin). A major question of developmental biology is when and how cell fates are determined during development. This is intimately related to the question of how pattern formation occurs during development. The embryo must not only generate the right number and type of differentiated cells, but they must be organized in the correct way relative to all the other cells in the embryo to form a functional animal. We will examine two possibilities of cell fate determination and pattern formation: 1. Cell fate could be determined by intrinsic factors placed into the egg during oogenesis and then parceled out to specific blastomeres during cleavage, 2. Extrinsic signals provided by the embryo's environment might provide the patterning information to regulate cell fate. As we will see most complex organisms use a combination of intrinsic and extrinic signals to regulate cell fate and embryonic pattern formation.
Autonomous cell fate specification by cytoplasmic determinants suggests that a cell's fate is entirely dependent on its lineage, whereas "regulative" development suggests that a cell's fate is determined by external signals from other cells. These two mechanisms of cell specification can be distinguished experimentally by isolation, ablation, and transplantation experiments. If a blastmere isolated from an embryo differentiates normally (as if it were still in its normal position in the embryo) we can say that it must have intrinsic determinants that specify its fate. However if it differentiates abnormally we can say that its cell fate is dependent on external signals. If we ablate a blastomere from an embryo and the embryo develops abnormally, missing all the cells fates that normally arise from the ablated blastomere, we say that development is cell autonomous and intrinsically specified. However, if the embryo develops normally we say that the remaining blastomeres can regulate their cell fate to compensate for the missing cells. If a transplanted cell maintains its cell fate based on its original position then we say its fate has been determined, if it takes on a new fate based on its newly transplanted position we say that its fate is regulated by external signals from nearby cells.
CYTOPLASMIC LOCALIZATION AND REGULATION IN THE TUNICATE EGG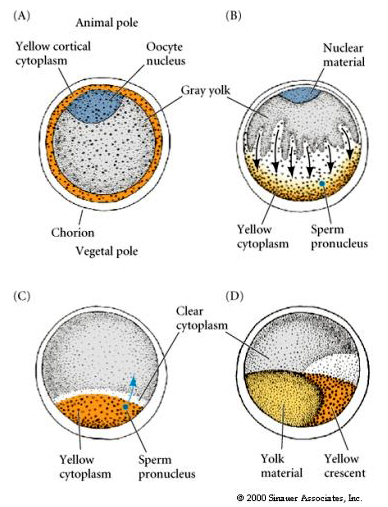
At the end of oogenesis the tunicate egg has a clearly distinguished animal and vegetal pole. There is a yellow cortical cytoplasm that surrounds a grey yolky inner cytoplasm. The oocyte nucleus is displaced towards the animal pole. Sperm entry in the vegetal hemisphere fertilizes the egg and initiates development. A dramatic rearrangement of the egg cytoplasm occurs after fertilization giving rise to regionally colored cytoplasms that seem to correlate with subsequent blastomere fates.
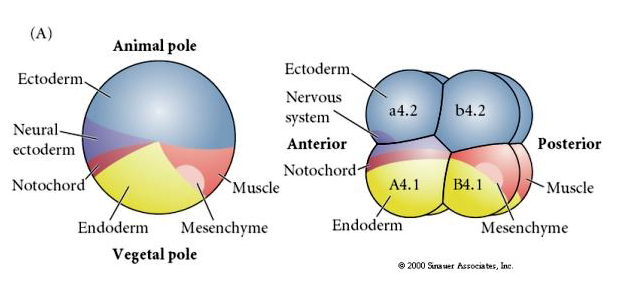 Note the fate map correlates with the different colored cytoplasms of the tunicate embryo. Don't be confused by the different colors in two figures. The "orange" yellow crescent cytoplasm is correlated with muscle fates and the Yolky (yellow) cytoplasm is correlated with endodermal fates. The grey (white or bluish purple) cytoplasm above the yellow crescent is correlated with neural ectoderm.
Note the fate map correlates with the different colored cytoplasms of the tunicate embryo. Don't be confused by the different colors in two figures. The "orange" yellow crescent cytoplasm is correlated with muscle fates and the Yolky (yellow) cytoplasm is correlated with endodermal fates. The grey (white or bluish purple) cytoplasm above the yellow crescent is correlated with neural ectoderm.
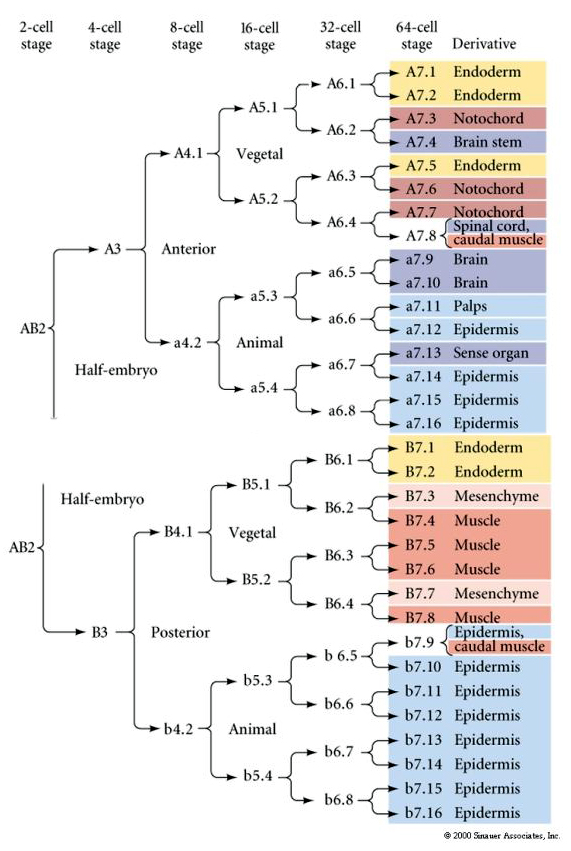 This lineage map shows the invariant linage correlation with blastomeres parceled particular colored cytoplasms by the invariant cell cleavages. However, invariant cleavages and lineages do not necessarily prove autonomous cell specification by cytoplasmic determinants.
This lineage map shows the invariant linage correlation with blastomeres parceled particular colored cytoplasms by the invariant cell cleavages. However, invariant cleavages and lineages do not necessarily prove autonomous cell specification by cytoplasmic determinants.
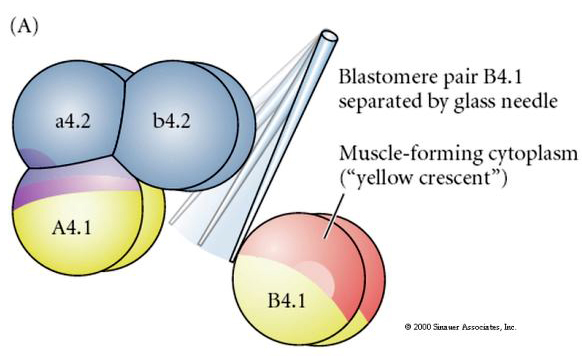 Experimental manipulations are required to test regulative versus cell autonomous determination of cell fate. The classic isolation experiments shown in the next three figures attempt to show that cell fate is determined by cytoplasmic determinants they acquire through stereotype cleavages. A glass needle is used to separate the B4.1 pair of blastomeres from the rest of the embryo. The B4.1 blastomeres normally acquire the yellow crecent cytoplasm correlated with muscle cell fate.
Experimental manipulations are required to test regulative versus cell autonomous determination of cell fate. The classic isolation experiments shown in the next three figures attempt to show that cell fate is determined by cytoplasmic determinants they acquire through stereotype cleavages. A glass needle is used to separate the B4.1 pair of blastomeres from the rest of the embryo. The B4.1 blastomeres normally acquire the yellow crecent cytoplasm correlated with muscle cell fate.
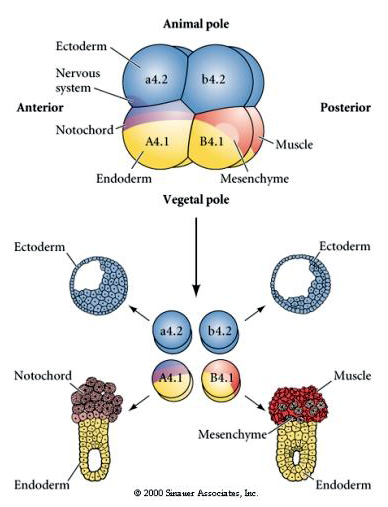
Here we can see the results of the isolation experiments. In each case the isolated blastomeres give rise to only that subset of cell fates they would normally produce in the intact embryo. The isolated blastomeres do not regulate their fate to compensate for their missing neighbors. Animal pole blastomeres, a4.2 and b4.2, give rise only to ectodermal cells. A4.1 gives rise to notochord and endodermal cells, while B4.1 gives rise to muscle and endodermal cells. None of the isolated blastomeres can give rise to all the cellular components of a normal embryo.
The next experiment below uses a needle to manipulate the equatorial cleavage plane so that it is more vegetal than normal and now the animal pole blastomeres, b4.2, acquire some of the "yellow crescent" cytoplasm. When these blastomeres are isolated they now give rise to some muscle cells. This nicely demonstrates that the "yellow crescent" cytoplasm can determine muscle cell fate and can do so in a cell autonomous manner.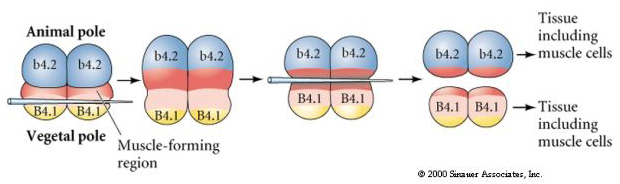
LOCALIZATION AND REGULATION IN THE SEA URCHIN EGG
A jelly canal defines the location of the animal pole and reflects the early polarity of egg. The early pattern of cleavages does not depend on the site of sperm entry, but are determined by the intrinsic polarity/asymmetry of egg. Boveri (1901) described a subequatorial band of pigment arranged orthongonally to animal-vegetal axis. These granules also indicated the location of cytoplasm that is later included in the cells of the archenteron. Horstadius (1928) separated animal and vegetal blastomeres and showed that only the vegetal blastomere would give rise to micromeres, gastrulate, and form skeleton. His conclusion was that cytoplasmic factors located in vegetal half are necessary for micromeres, gastrulation and archenteron fromation,and skeleton formation.
 Remember the pattern of early cleavages. The micromeres arise during the fourth cleavage (16 cell stage) from an unequal equatorial division of the vegetal pole blastomeres.
Remember the pattern of early cleavages. The micromeres arise during the fourth cleavage (16 cell stage) from an unequal equatorial division of the vegetal pole blastomeres.
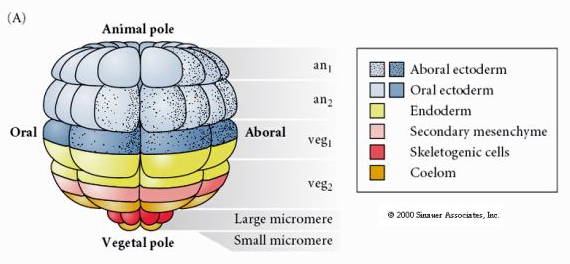 This shows the fate map of the 64 cell stage sea urchin blastula. Notice that the micromeres are the primary mesenchyme cells and give rise to the larval skeleton (the pluteus stage spicules).
This shows the fate map of the 64 cell stage sea urchin blastula. Notice that the micromeres are the primary mesenchyme cells and give rise to the larval skeleton (the pluteus stage spicules).
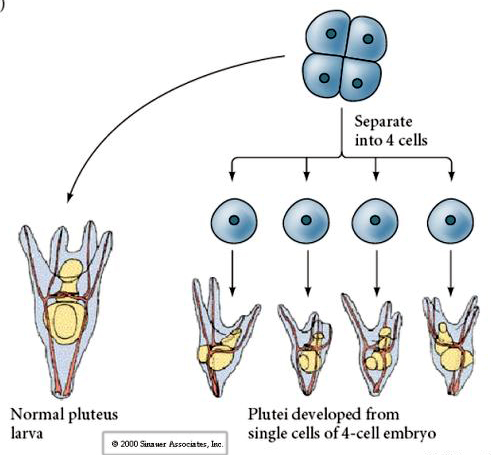 At the four cell stage, if the blastomeres are isolated from each other they are able to "regulate" their fate and give rise to 4 small pluteus stage larvae.
At the four cell stage, if the blastomeres are isolated from each other they are able to "regulate" their fate and give rise to 4 small pluteus stage larvae.
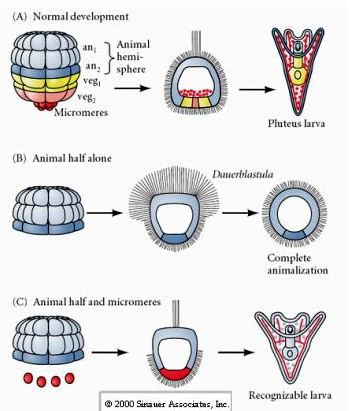 In contrast, at later stages if you isolate animal half blastomeres you find that they only produce an "animalized" dauerblastula that does not express any mesodermal or endodermal cell fates. Isolated vegetal half blastomeres give rise to larva that express ectodermal, mesodermal, and endodermal cell fates showing that the fate of these cells can be regulated. Isolated micromeres (primary mesenchyme) undergo the correct number of cell divisions and ALWAYS give rise to spicules on schedule. Thus, micromeres are definitively specified as the precursors of the skeletogenic mesenchyme cells when they first appear at the 16 cell stage. The key experiments were putting micromeres together with animal pole blastomeres and showing that although micromere fate was "fixed or determined" at the time of their birth, micromeres were able to "induce" new cell fates in the animal pole blastomeres. The micromeres were able to induce endodermal and mesodermal fates in the animal pole blastomeres! Thus, the late experiment in "C" shows that when micromeres are added to an animal half blastula you can now induce the formation of a recognizable larva expressing endodermal, mesodermal, and ectodermal fates.
In contrast, at later stages if you isolate animal half blastomeres you find that they only produce an "animalized" dauerblastula that does not express any mesodermal or endodermal cell fates. Isolated vegetal half blastomeres give rise to larva that express ectodermal, mesodermal, and endodermal cell fates showing that the fate of these cells can be regulated. Isolated micromeres (primary mesenchyme) undergo the correct number of cell divisions and ALWAYS give rise to spicules on schedule. Thus, micromeres are definitively specified as the precursors of the skeletogenic mesenchyme cells when they first appear at the 16 cell stage. The key experiments were putting micromeres together with animal pole blastomeres and showing that although micromere fate was "fixed or determined" at the time of their birth, micromeres were able to "induce" new cell fates in the animal pole blastomeres. The micromeres were able to induce endodermal and mesodermal fates in the animal pole blastomeres! Thus, the late experiment in "C" shows that when micromeres are added to an animal half blastula you can now induce the formation of a recognizable larva expressing endodermal, mesodermal, and ectodermal fates.
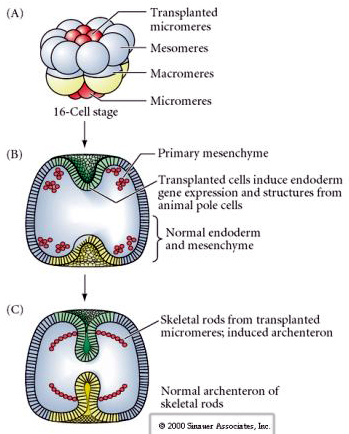 The final set of experiments demonstates that even in a normal embryo, if you transplant micromeres to the animal pole cap you can induce a secondary archenteron and alter the normal axial patterning. This again argues that the micromeres acquire a cytoplasmic derminant the specifics their cell fate and that they provide the inductive signal that patterns the axial structures of the sea uchin embryo. Micromere fate cannot be altered, but signals from the micromeres can alter the fate of all the other blastomeres.
The final set of experiments demonstates that even in a normal embryo, if you transplant micromeres to the animal pole cap you can induce a secondary archenteron and alter the normal axial patterning. This again argues that the micromeres acquire a cytoplasmic derminant the specifics their cell fate and that they provide the inductive signal that patterns the axial structures of the sea uchin embryo. Micromere fate cannot be altered, but signals from the micromeres can alter the fate of all the other blastomeres.
Horstadius: (1928, 1935) showed experimentally that in a 16 cell stage embryo all tiers of blastomeres except the micromeres will take on different fates when transplanted into different positions in chimeric embryos. The archenteron will develop from veg 1 blastomeres if veg 2 cells are removed and the micromeres are placed in contact with the veg 1 layer. In the absence of micromeres, veg 2 blastomeres give rise to archenteron and skeletal structures. Classically, a duel animal-vegetal gradient has been invoked to account for these results. However these results only indicate that decisive inductive interactions occur between adjacent blastomere tiers.
Implanted individual micromeres near the animal pole inhibit apical tuft formation and in some cases induce a new embryonic axes. Veg 2 blastomeres will also induce changes similar to micromeres when transplanted next to animal pole blastomeres.
GENERAL RESULT OF TRANSPLANTATIONS: the fate of given blastomeres is always found to be affected by the apposition of different neighboring cells that adjoin them in normal embryos.
HYPOTHESIS: Localized maternal cytoplasmic determinants specify certain cells in the normal embryo, in particular the micromeres and the archenteron precursors near the vegetal pole. These cells then determine inductively the fates of neighboring blastomeres, which interact in turn with their neighbors. Many of the blastomeres retain potentialities other than those they normally express, and for some time these blastomeres are only reversibly specified, as required for a developmental system that depends to a large extent on induction.