|
|
DEVELOPMENTAL BIOLOGY 3230 |
|
|
| Neurogenesis |
|
|
|
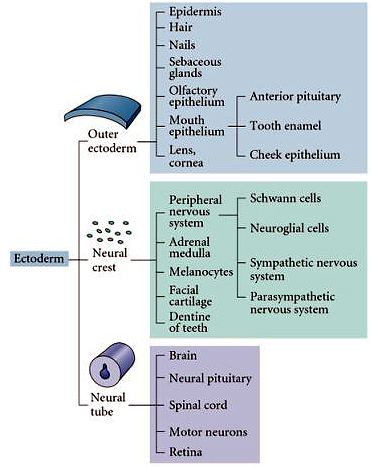
The ectoderm gives rise to three primary tissues: the Outer ectoderm that gives rise to the epidermis, the Neural Crest that gives rise to the peripheral nervous system, facial cartilage, and melanocytes, and the Neural Tube that gives rise to the central nervous system. A more complete listing of the ectodermal derivatives is listed at right. Remember that the ectoderm is initially patterned by the gradients of BMP-4 and Wnt that act as "anti-neuralizing" agents. The ectoderm's default differentiation is neural, so the opposing gradients from the organizer (dorsal mesoderm) of follistatin, chordin, noggin (block BMP function) and FrzB, dickkopf, and cerberus (block Wnt function) act to neuralize the ectoderm. Thus the dorsal-medial ecotoderm that lies above the foregut endoderm and the dorsal mesoderm becomes the neural plate. The neural plate folds up to form the neural tube. The epedermal ectoderm is brought together by the lateral folds of the neural tube at the dorsal midline and fuses to complete the outer epidermal covering of the embryo. The openings at the anterior and posterior ends (anterior and posterior neuropore) must also close and be covered by epidermal ectoderm. This is just the beginning of neurogenesis. The neural tube is highly patterned along both the anterior-posterior and dorso-ventral axes. The brain at the anterior end of the neural tube is a dramatic expansion of highly patterned neural structures unique to vertebrates and uniquely expanded in humans. |
 |
|
|
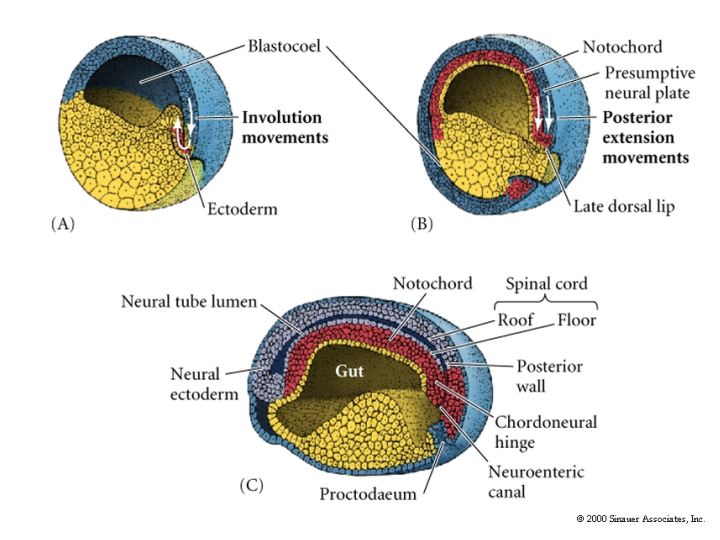
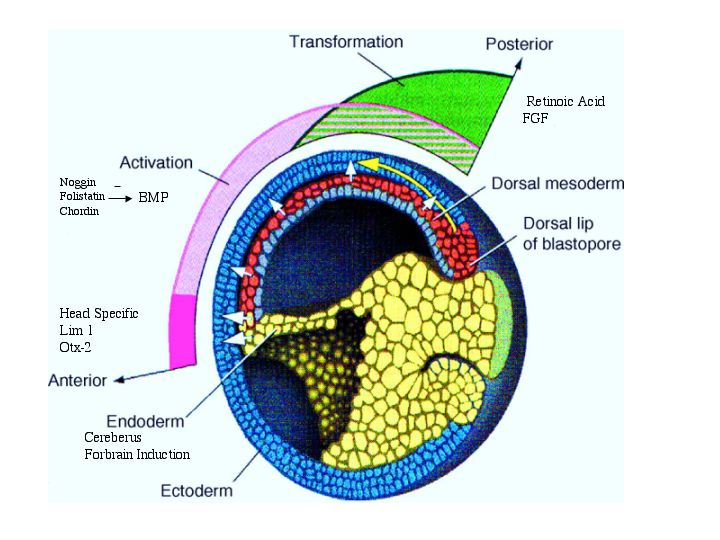
Review the figure at right showing the positions of the dorsal mesoderm after gastulation. The most dorsal mesoderm that induces neural plate is first called chordamesoderm. The chordamesoderm gives rise to the prechordal plate, notochord, and dorsal mesoderm (axial mesoderm that gives rise to the somites). Chordamesoderm is the source of follistatin, chordin, and noggin, and Frzb and dickkopf, that antagonizes the BMP and Wnt gradients respectively, from the ventral mesoderm and endoderm. Also remember that the foregut endoderm expresses cerberus that is also an important inhibitor of BMP and Wnt in the the most anterior region of the neural plate. There is also posterior to anterior gradients FGF and retinoic acid that are thought to be from the dorsal lip (organizer). The main derivative of the chordamesoderm that is important for patterning the dorso-vental axis of the neural tube is the notochord. |
 |
|
 |
|
|
|
 |
|
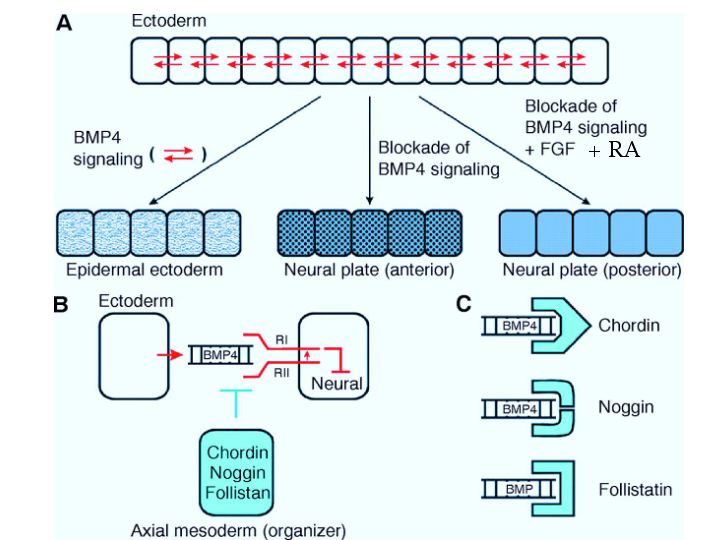
| The figure at right reviews the role of chordin, noggin, and follistatin in the formation of the neural plate. It also suggests that some anterior-posterior patterning is due to gradients of FGF and retinoic acid from the organizer. |
|
| The figure at right shows an amphibian embryo at the neurula stage. Note the positions of the neural plate and neural folds as the neural tube forms. Note the positions of the anterior and posterior neuropores. |
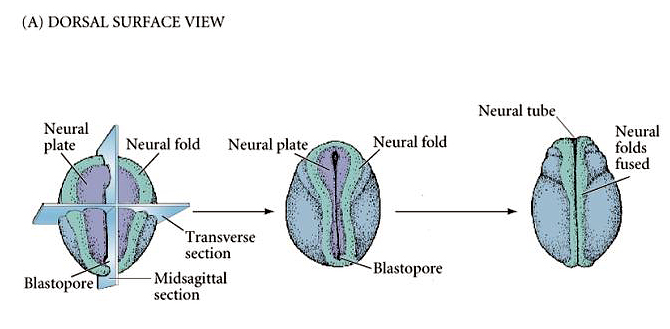 |
|
| Human neurulation occurs in a similar fashion. Note that correct closing of the anterior and posterior neuropores is a big deal! If the neural tube does not close at its dorsal margin, or at the two ends, it results in a severe birth defects. Most spontaneously aborted embryos show gross defects in neurulation. Note that anencephaly developes if the anterior neuropore fails to close. Spinal bifida develops if the neural tube fails to close more posteriorly along the neural axis. |
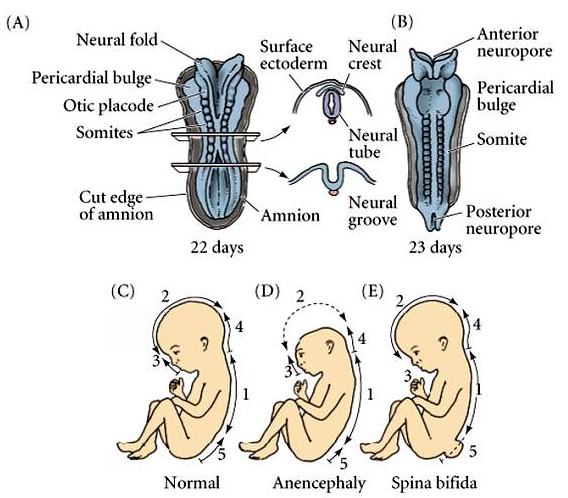 |
|
|
|
The illustration at right shows the medial-lateral fate map of the nerual plate and the shape changes associated with neuralation. Notice that the neural crest arises from the lateral most neural ectoderm. |
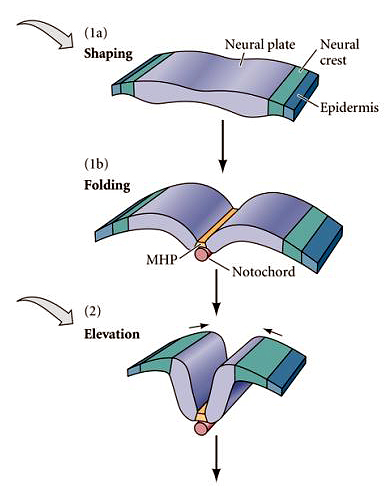 |
|
| See Reviews of neural tube defects and role of folic acid. |
|
|
|
|
|
|
The forces driving neurulation include both intrinsic shape changes of cells within the neural plate and forces derived from adjacent epidermal ectoderm. Note the appearance of the notochord at the ventral midline of the neural tube. The notochord forms from the chordomesoderm. |
|
| The anterior posterior and dorsal ventral patterning is already taking place during these early stages of neurulation. Note that the notochord is an important inductive tissue for ventral patterning of the neural axis. |
|
| The lateral edges of the neural folds come together and fuse at the dorsal midline. |
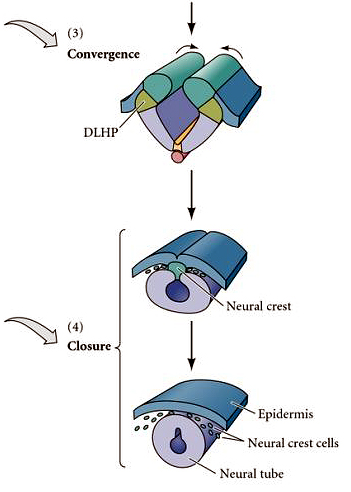 |
|
| The epidermal ectoderm fuses at the midline to complete the epidermal covering of the embryo. The neural crest arises at the dorsal midline of the neural tube and migrates throughout the body to give rise to all the peripheral nervous system and many other important structures in the head. The neural tube completely separates from the epidermal layer. What drives segregation of neural, crest, and epidermal cells? One common feature that is correlated with cell segregation during development is the expression of unique combinations of cell adhesion molecules. |
|
| A simple experiment shown at right illustrates that when cells from different tissues are dissociated and then mixed together they have the ability to sort out and reaggregate. This ability to recognize and associate with other cells from the same tissue has been linked to the expression of unique combinations of cell-cell adhesion molecules. |
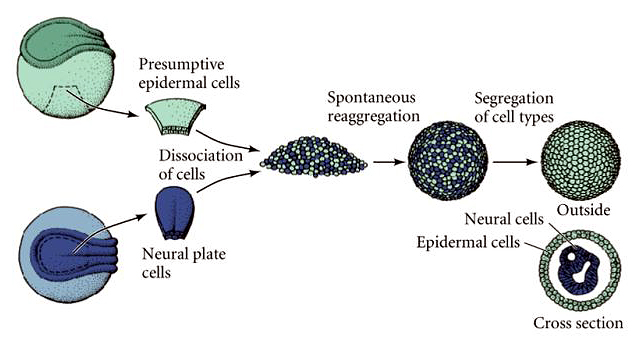 |
|
|
 |
|
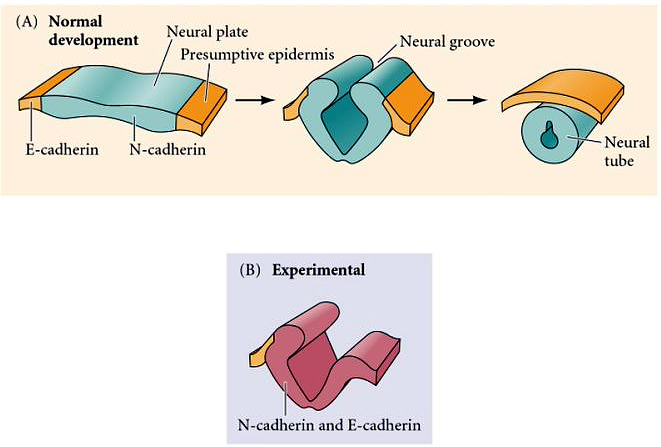
| Initially, all the ectoderm expresses a calcium dependent homophilic adhesion molecule called E-cadherin. The induction of the neural plate and its separation from the epidermal ectoderm is correlated with the down regulation of E-cadherin and the up regulation of two new cell adhesion molecules, N-cadherin and NCAM. You can show experimentally that N-cadherin is important for the segregation of neural plate from epidermal ectoderm. If you cause the nearby epidermal ectoderm to express N-cadherin it segregates with the neural plate tissue during neurulation and becomes incorporated into the neural tube. |
|
| Some have argued that the evolution of the brain was a major evolutionary milestone. An anterior neural specialization evolved very early in animal evolution as you will see when you compare the molecular specification of the fly and mouse brain. The human brain is a major expansion and specialization of the anterior neural tube. At right you can see the major divisions and derivatives of the anterior neural tube. The anterior specializations of the neural tube are initiated early in neurulation by the morphogen gradients from the organizer and the ventral endoderm and mesoderm. |
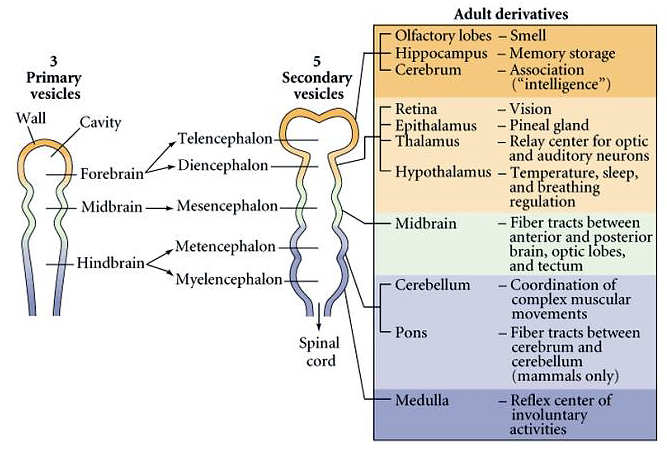 |
|
|
 |
|
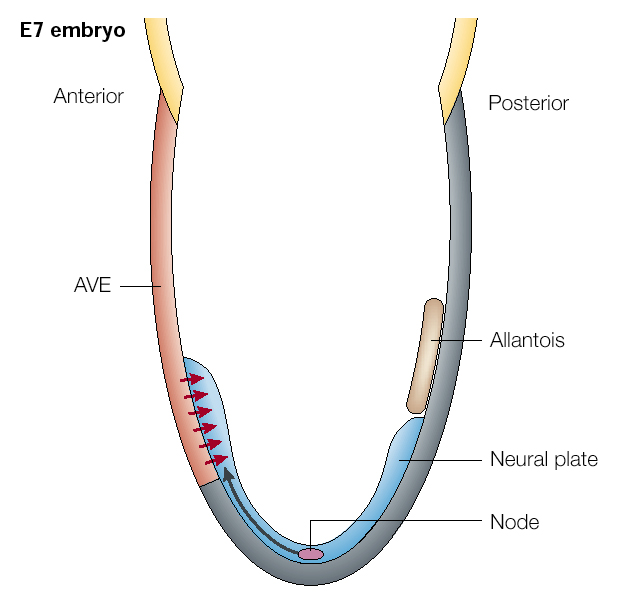
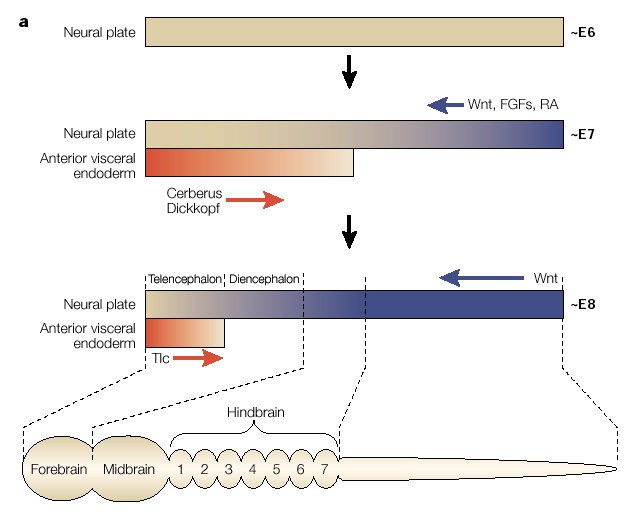
| At right is a mouse embryo at E7. The node is the primary organizing center (equivalent to Hensen's node and the dorsal lip of the organizer in amphibians). It is the source of the FGF and RA gradients that posteriorize the neural axis. It is also the source of BMP and Wnt inhibitors (follistatin, noggin, chordin, Frzb, dickkopf). Note that in mammals it is the anterior visceral endoderm (extraembryonic endoderm) that is the source of the inductive signal responsible for "brain" induction (red arrows). The AVE secretes cerberus and dickkopf that antagonizes the a effects of Wnt and BMP. |
|
|
|
 |
|
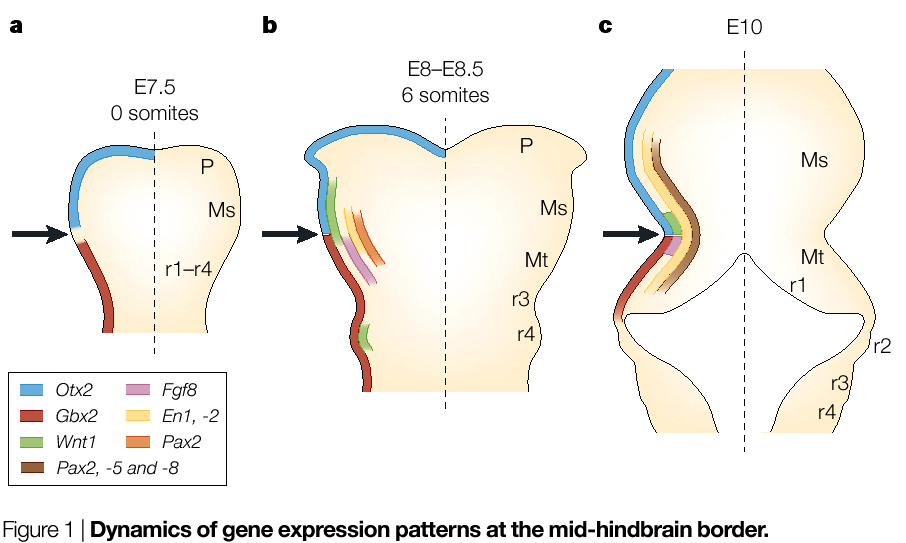
| Soon after the neural plate is formed through the inhibition of the BMP, the gradients of Wnt, FGF, and RA posteriorize the neural axis. The anterior visceral endoderm lies just under the anterior most neural plate and secretes cerberus and dickkopt that inhibits Wnt and BMP to "anteriorize" the neural tube. Later, Tlc is secreted and also antagonizes Wnt function. This further regionalizes the anterior most region of the neural into telencephalon. Note that the hindbrain is divided into segment-like compartments called rhombomeres (1-7). We will discuss how the hindbrain "segements" are specified later. First I want to focus on another early morphological marker of patterning. The isthmus is a constriction that arises at the midbrain-hindbrain junction. It is one of the earliest morphological markers and for that reason has been extensively characterized. |
|
|
 |
|
|
|
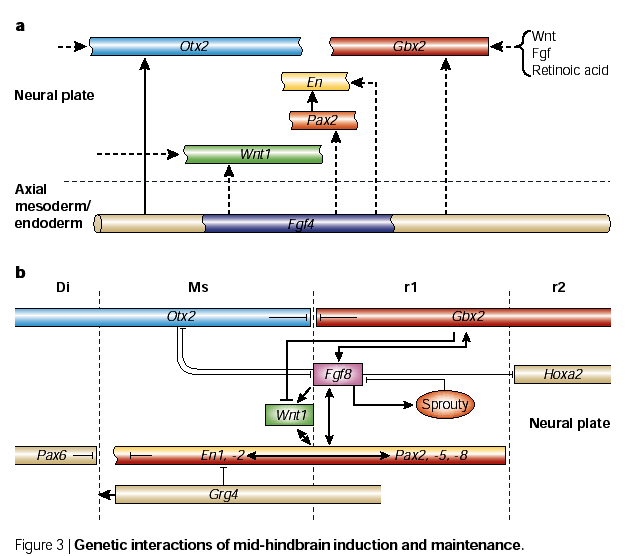
Transplantation studies showed that the isthmic region behaved as an "organizer". When transplanted to other locations along the neural axis it caused duplications of midbrain-hindbrain pattern elements. Again, rapid progress started when homologs of important fly genes regulating patterning were found expressed at the isthmus. There are two mouse homologs of the fly engrailed gene, en-1 and en-2. Both of these are expressed early at the isthmus region. En-1 is expressed more anteriorly from the isthmus and En-2 more posteriorly. |
|
| A mouse KO of En-1 lacks a midbrain, while a mouse KO of En-2 lacks a normal cerebellum (a hindbrain derivative). Interestly, En-1 and En-1 proteins are functionally interchangable, it is the regulatory regions that are unique. How is Engrailed induced at the isthmus? The signal is probably Fgf4 secreted by axial mesoderm or endoderm (depending on the species). Fgf4 induces the two major morphogens at the isthmus, Wnt-1 and Fgf8. These two morphogens are responsible for the major patterning of the midbrain-hindbrain region. You can see at right that the interactions among the gene products of the isthmic organizing center are very complex; they establish and maintain morphogen gradients (Wnt-1 and Fgf8) and sharp boundarys of gene expression. Otx2 is antother homeodomain containing transcription factor that specifies forebrain. Mouse Otx2 KOs (and Lim-1) have not anterior brain, but hindbrain and spinal cord are relatively normal. Remember that Otx2 and Lim-1 are induced by cerberus, dickkopf, and Tlc. Notice that Otx2 and Gbx2 inhibit one another. This maintains the sharp border of Fgf8 and Wnt1 because Otx2 inhibits Fgf8, while Gbx2 inhibits Wnt-1. |
|
|
 |
|
|
|
 |
|
|
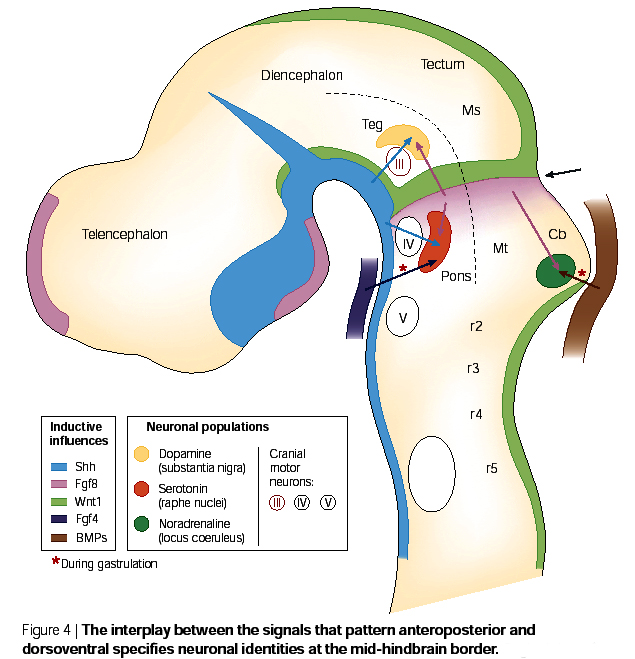
The major morphogen gradients patterning the anterior-posterior and dorsal-ventral axes of the mammalian brain are shown at right. Note that the isthmus is a major anterior-posterior organizing center and express Wnt-1 and Fgf8. There is also a more anterior "telecephalon" organizing center that expresses Fgf8. Notice that Shh is expressed at the ventral midline and BMP at the dorsal midline. These two morphogens are important for the dorsal ventral patterning of the brain. |
|
|
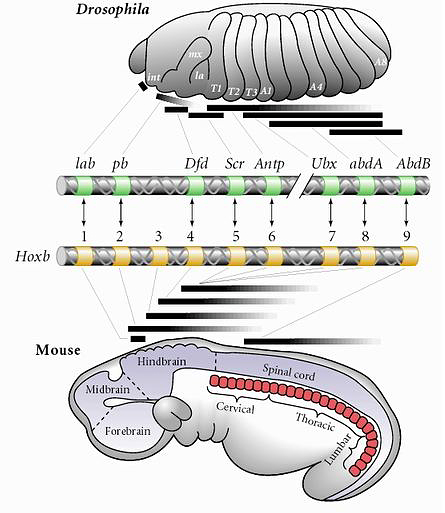
The next qestion is how are these morphogen gradients interpreted to specify axial identity? Again, the Hox genes are key players. Remember that the Hox genes are expressed along the body axis in remarkably similar patterns in the fly and the mouse. Remarkably, this is also true for the central nervous sytems of the fly and mouse. |
|
 |
|
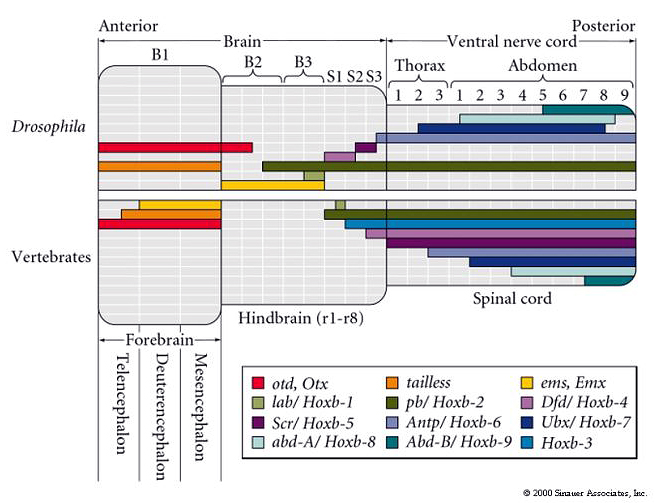
| The expression of Hox and other homeodomain containing transcription factors is eerily similarly in the fly and the mouse brain! This simillarity has led to very rapid progress in understanding how the human brain is regionally specified during neurogenesis. One surprising finding is that the morphogen gradients that specify Hox gene expression are different even though the final pattern is similar. The other point is that Hox genes are obviously not unique to "segmented" domains, but are used throughout the animal kingdom to regionally specify identity. One of the first places developmental biologists looked to see if Hox genes were functioning in a similar way in fly and mouse was the hindbrain. The hindbrain has a repeated structure that is the only region of the brain that looks "segmented". |
 |
|
|
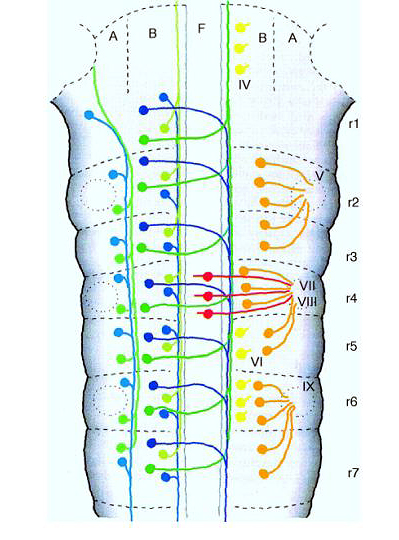
Remember that early in brain development you can see hindbrain "repeat units" termed rhombomeres. Seven rhombomeres develop that share many features. Each has a repeated pattern of interneurons, motorneurons, and sensory neurons (derived from neural crest), but each rhombomere also has unique features that allow one to easily identify it. Thus the seven rhombomeres (r1-7) look like a classic case of "segmentation". In fact each rhombomere develops from compartments that behave just like the AP compartments seen during fly development. Cells within a compartment do not mix with cells from adjacent compartments and Hox gene expression is respects compartment boundaries. |
 |
|
|
|
|
|
 |
|
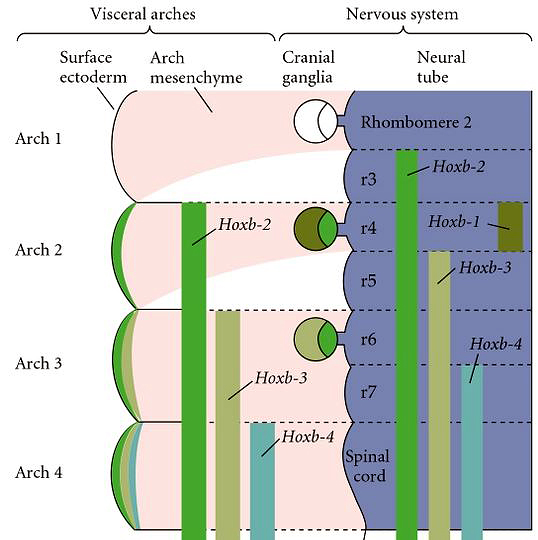
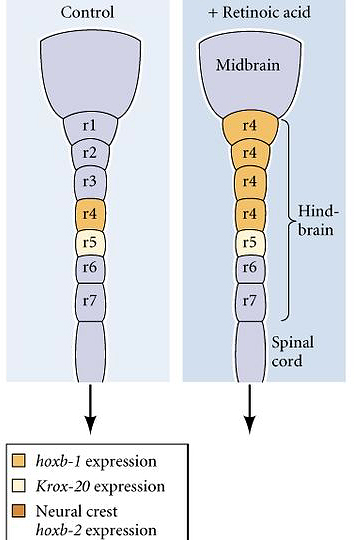
| The major question that was first asked about Hox genes and vertebrate axis specification was whether mutations in Hox genes caused homeotic transformations like Hom gene mutations in Drosophila. This was first addressed in the hindbrain because of the similarity between rhombomeres and fly segments. Note that the Hoxb-1, 2, 3, and 4 genes have sharply defined anterior boundaries that correspond to the anterior boundaries of rhombomeres 3,4,5, and 7. |
|
| Remember that many Hox genes are retinoic acid response elements that regulate their expression. Hoxb-1 is normally expressed in rhombomere 4, but if you experimentally increase the concentration of retinoic acid during neurulation you can cause an anterior expansion of the hoxb1 domain of expression. As the hindbrain develops you can see that the anterior expansion of Hoxb-1 respecifies the identity of rhombomeres 1,2, and 3 to a rhombomere 4 identity. This was the first indication that Hox genes specifiy identity in vertebrates in much the same way as in Drosophila. Since these early experimental manipulations, gene targeting by homologous recombination in mouse has become routine and each of the mouse Hox genes has been targeted. There is an extensive literature developing on the Hox code specifying different regions of the developing nervous system. I have included one paper by Capecchi that describes mutations in Hox 1 and 2 genes that regulate hindbrain development. |
 |
|
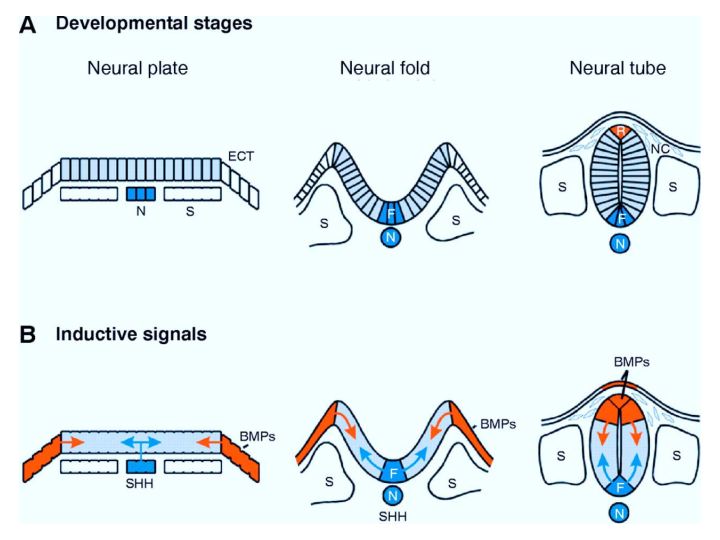
| Anterior-posterior and dorso-ventral patterning begin during neurulation. In B you can see that the inductive signals used to pattern the dorso-ventral axis are present at the neural plate stage. The chordomesoderm forms the notochord. The notochord is a source of sonic hedgehog that induces a floor plate at the ventral midline of the neural tube. The floor plate then secretes sonic hedgehog that is the essential ventral morphogen. The dorsal epidermal ectoderm (note that it is initially at the lateral edge of the neural plate) is a source of BMPs that induce the formation of a roof plate in the neural tube. The roof plate then becomes a source of dorsalizing morphogens that includes several members of the TGFbeta family of growth factors (BMPs, Dorsalin, Activins) |
 |
|
|
|
|
 |
|
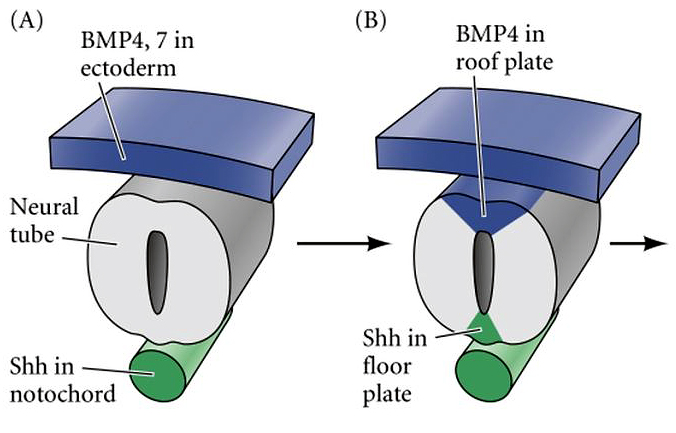
| The schematic at right shows (A) the inductive Shh coming from the ventral notochord and the BMP4,7 coming from the epidermal ectoderm overlying the neural tube; (B) the dorsal BMPs induce a roof plate structure that expresses BMP4, while notochord Shh induces a floor plate structure that secretes Shh. |
|
|
 |
|
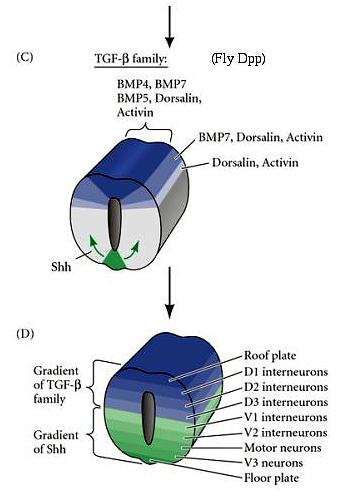
| (C) The roof plate secretes several members of the TGF-beta family of growth factors that establish dorsal to ventral morphogen gradients. At the same time secreted Shh from the floor plate establishes an opposing ventral to dorsal gradient. |
|
| (D) Notice how the roof plate TGF-beta family gradients and the floor plate Shh gradient are interpreted by cells along the dorso-ventral axis to specify their neuronal identity. Generally speaking, neurons involved in processing sensory information are located dorsally and motor output (motor neurons) located ventrally. |
|
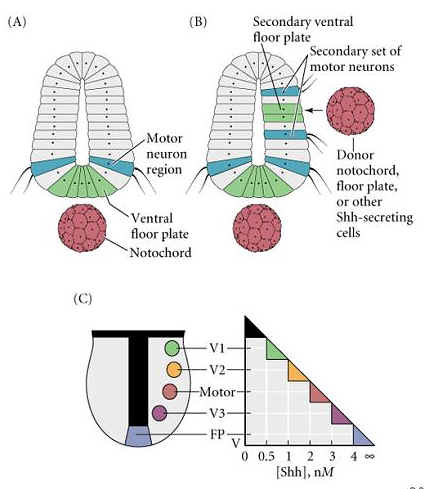
| Experimental evidence supporting the role of a Shh morphogen gradient in specifying ventral neuronal identity is shown at right. Transplanting an ectopic notochord to the side of the neural tube causes a duplication of the floor plate and the most ventral motor neuron pools. You get the same results from transplanting a bead containing Shh to the lateral edge of the neural tube. In (C) a model is presented that shows how threshold values of Shh could differentially specify the identity of neurons in the ventral region of the neural axis. |
|
 |
|
| THE CURIOUS CASE OF THE CLYCLOPS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

















































