|
|
|
DEVELOPMENTAL BIOLOGY 323O |
|
|
| Fly Dorso-Ventral Polarity |
|
Conserved Pathways dpp |
|
|
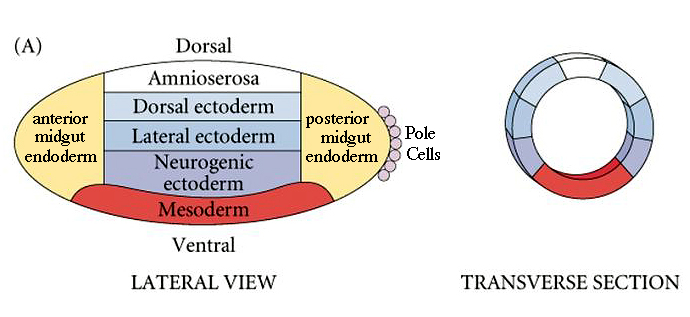
| Fly dorso-ventral polarity is established by maternal genes expressed in the oocyte and nurse cells and follicle cells surrounding the developing oocyte. We can draw a dorso-ventral fate map, just as we could draw an anterior-posterior fate map. Note that the mesoderm is at the ventral midline and the ectodermal derivatives are sequenctially more dorsal. The neurogenic ectoderm ends up at the ventral midline after gastrulation. |
 |
|
| A saturation mutagenesis was performed to identify all genes involved in establishing the dorso ventral pattern in Drosophila. Just as with the screen for A-P pattern mutants, the cuticle pattern could be used to identify mutants. Two important mutants that were identified were Toll and Dorsal. These mutants were severely dorsalized (missing all ventral pattern structures). A surprising finding was that cytoplasm from ANYWHERE in a wild type egg could rescue these mutations. This was not expected because the region of activity for the Toll and Dorsal proteins was presumed to be just the ventral region. The prediction was that Toll and Dorsal would be expressed as morphogen gradients with the highest concentration ventrally. When the Dorsal and Toll proteins were identified it was shown that they are present everywhere in the early embryo. However, Toll is a receptor on the early embryo plasma membrane and it is only activated by a ligand secreted by the ventral follicle cells. Activated Toll causes the "inactive" Dorsal protein to be activated and migrate to the nucleus in a ventral to dorsal gradient. |
|
|
|
|
 |
|
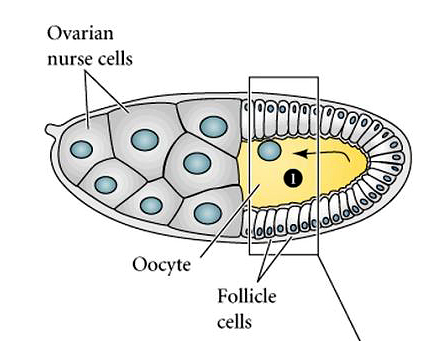
| In the figure at right you can see the relationship of the nurse and follicle cells to the developing oocyte. The oocyte nucleus migrates anteriorly and dorsally to lie just beneath the dorsal follicle cells. Initially, the follicle cells are symmetric, but under the influence of the oocyte nucleus the dorsal follicle cells are given a dorsal cell fate. |
|
|
 |
|
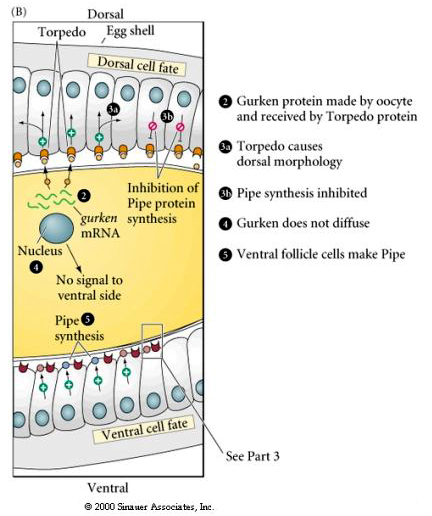
| The figures at right illustrate the molecular signalling pathway that leads to the dorsal translocation into the nucleus of the ventral follicle cells. Locally secreted Gurken binds to the Torpedo receptor on the follicle cells and induces dorsal follicle cell fate. This inhibits the synthesis of Pipe protein by the dorsal follicle cells. The ventral follicle cells make Pipe and this leads to Toll activation just in the ventral region of the embryo. |
|
|
|
 |
|
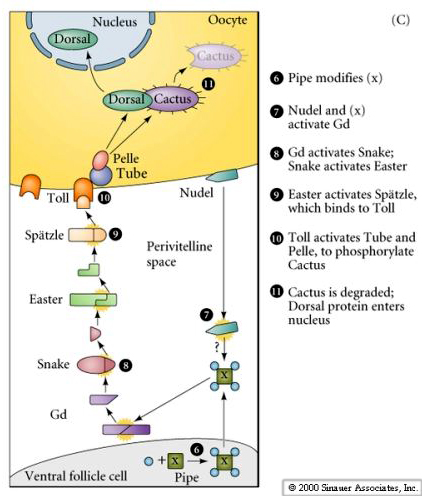
| Pipe protein initiates a signalling pathway that leads to the production of activated Spatzle just in the ventral perivitelline space (this is the space between the follicle cells and the egg plasma membrane). Spatzle binds to Toll and Toll activates the Pelle kinase. Dorsal is bound by Cactus, which blocks Dorsal's nuclear localization signal. The Pelle kinase phosphorylates Cactus and causes it to release the Dorsal protein. Cactus is degraded. Dorsal is now free to migrate to the nucleus where it will act as a transcriptional activator of the ventralizing genes (snail, twist, and rhomboid) and a transcriptional repressor of the dorsalizing genes (dpp, tolloid,and zerkullt) |
|
|
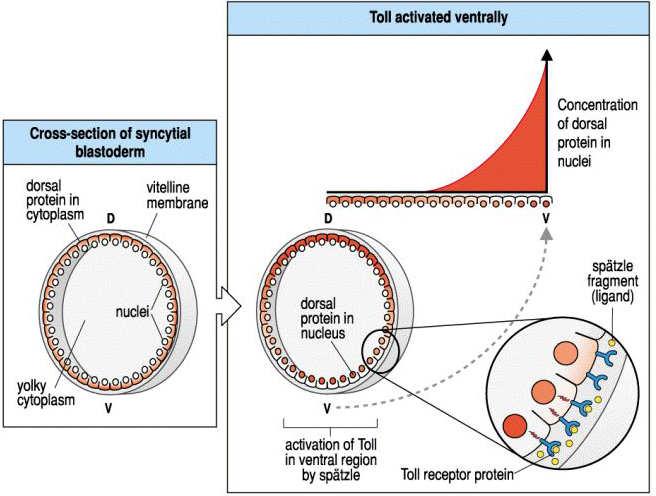 |
|
| In the early syncycial blastoderm you can see that Dorsal protein is uniformly localize to the egg cytoplasm. However, activation of Toll by the ventral follicle cells causes a gradient of Dorsal translocation to the nucleus. By late syncycial blastoderm or early cellular blastoderm you can see a concentration gradient of the Dorsal protein in the nuclei of cells along the dorso-ventral axis. |
|
|
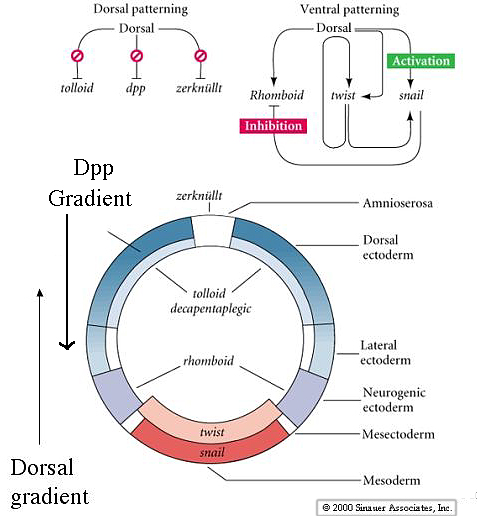 |
|
| The gradient of nuclear Dorsal protein acts to repress the dorsalizing genes Dpp, tolloid, and zerknullt in the ventral region. In the dorsal zone these dorsalizing genes are autonomously expressed and establish important gradients that oppose the actions of Dorsal. The gradient of Dpp is esential for dorsal pattern elements and specifies the amnioserosa and dorsal ectoderm. Dorsal activates twist, snail and rhomboid in the ventral zone. Notice how the inhibitory interaction between snail and rhomboid prevents rhomboid from being expressed at the ventral midline. Twist and snail specify the mesoderm, while rhomboid specifies the neurogenic ectoderm at a more lateral and dorsal location. |
|
|
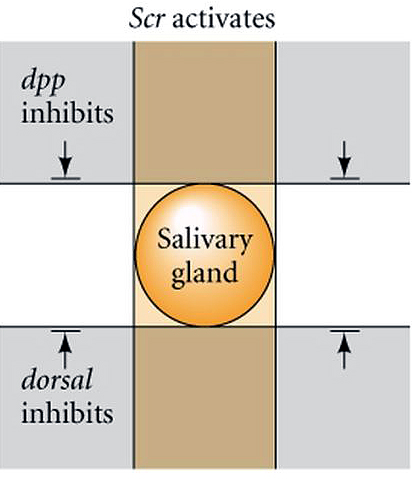 |
|
|
| How are the anterior-posterior and dorsal-ventral axis coordinated? Clearly segment specific structures develop at specific dorsal-ventral locations. The positioning of salivary glands is a good illustration of this. Salivary glands are only found associated with the first thoracic segment. The first thoracic segment is specified by the Scr homeotic gene of the antennapedia complex. When you misexpress Scr in additional segmental domains you find that the salivary gland is duplicated. The salivary gland always develops midway between the dorsal and ventral midline. If you misexpress dpp and dorsal you find that the salivary gland is inhibited by both. You can cause the salivary gland to form either too far ventrally or too far dorsally by altering the gradients of dorsal or dpp. Thus, the exact anterior-posterior and dorsal-ventral position of structures can be determined by the coordinated response to the segment specific homeotic genes and the gradients of Dpp and Dorsal. |
|
| Although the Dorsal signaling pathway in fly might seem complex and esoteric, in fact it is a highly conserved pathway that is used in mammals to regulate several important developmental and cellular functions. Not only is it used in fly development, but again later to regulate the fly's response to bacterial invasion. Here's an example of the evoloutionarily conserved pathway that regulates both the fly and mammalian immune function. Boxes of the same color represent molecular "homologs" in Drosophila and Mammals. |
|
|
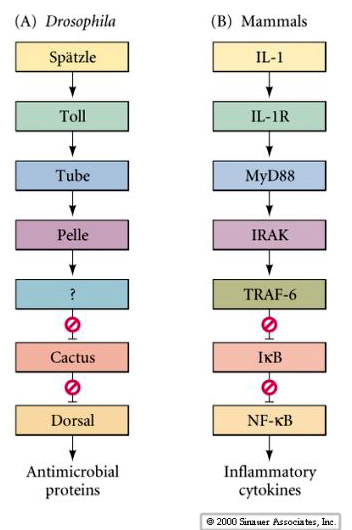 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|















