|
|
|
|
DEVELOPMENTAL BIOLOGY 3230 |
|
|
| Zygotic Genes regulating segmentation |
|
|
|
|
 |
|
| The cytoplasmic polarity genes regulate the expression of the zygotic genes. We generally present this as a hierarchical and sequential pathway from cytoplasmic polarity to GAP to Pair Rule to Segment Polarity and Homeotic genes where at each subsequent level the regional pattern specification becomes finer and more complex. However, keep in mind that it is a regulator network that relies on feedback and feed forward interactions to assure the proper localization and sharp borders of gene expression. Also remember that gene expression can become independent of the original activating signals.
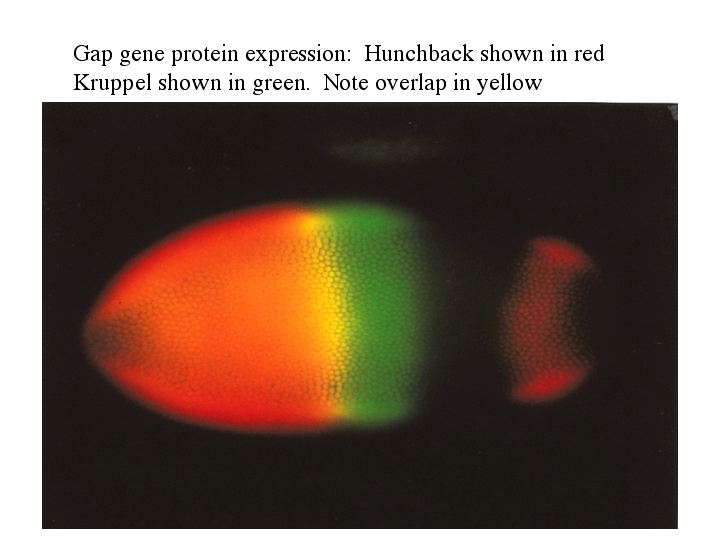
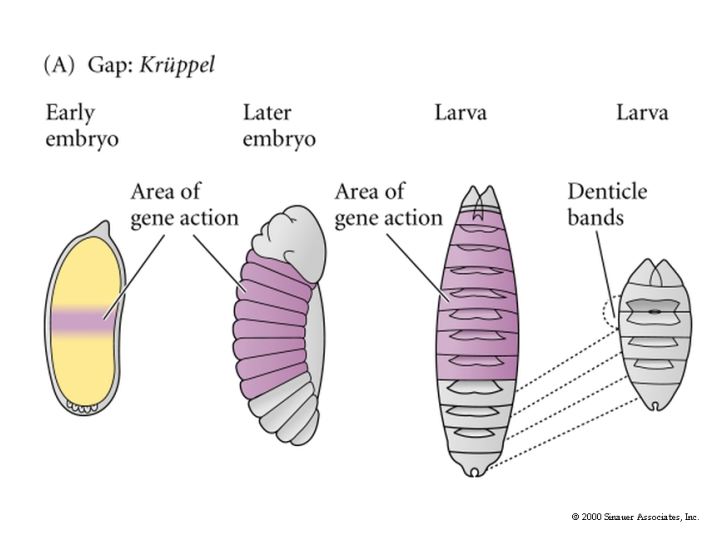
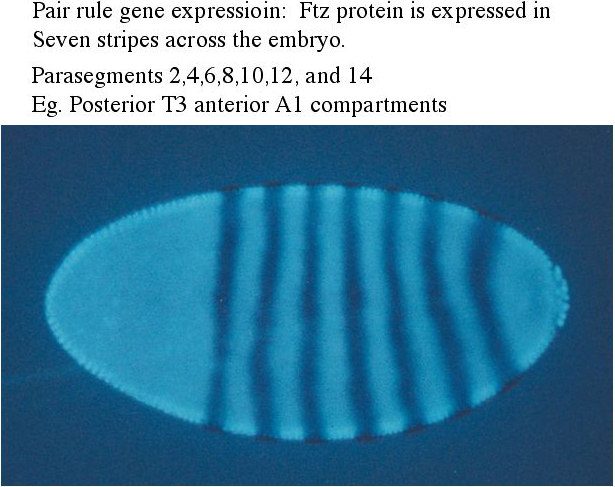
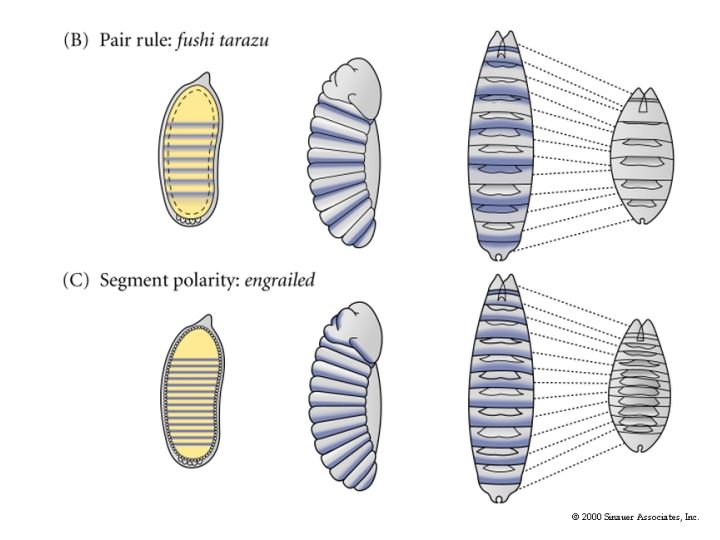
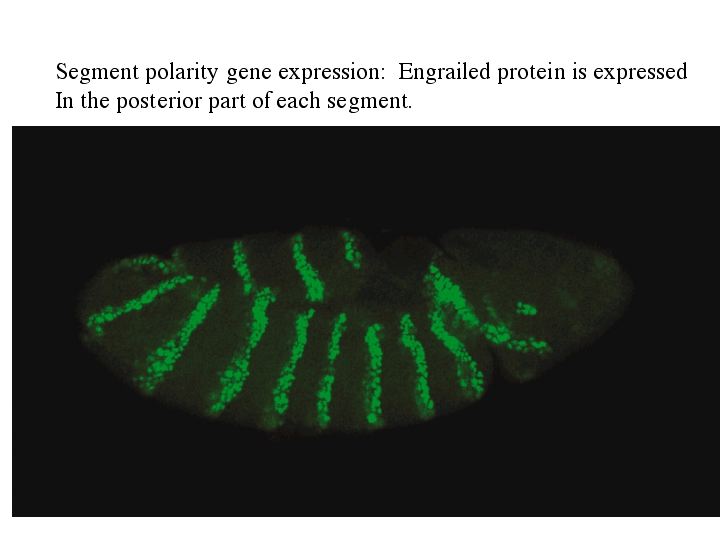
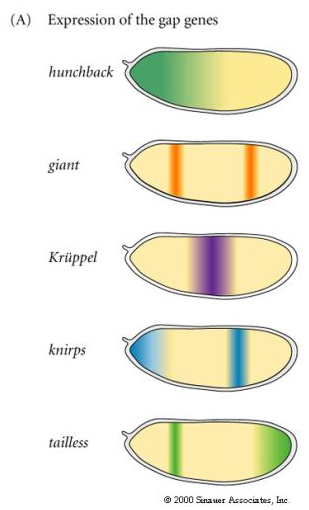
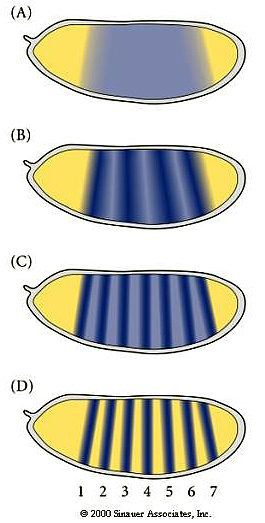
The cytoplasmic polarity genes broadly specify anterior, posterior and terminal regions. The information in these broad morphogen gradients are used to specify smaller regions of gene expression by the GAP genes. Mutations that resulted in the deletion of several adjacent segments were classified in the GAP category. Generally the area of gene action corresponded to the "missing" segments in the mutant animal. An example of this is shown at right. The GAP gene Kruppel is expressed in middle region of the developing embryo fated to give rise to the anterior segments. In Kruppel mutants these anterior segments are missing. This pattern of the region of gene expression correlating strongly with missing pattern elements is also see in Pair Rule and Segment Polarity genes. Below you can see examples of Pair rule gene expression in 7 stripes, ftz, and the resulting pattern defects seen in every other segment of the larval cuticle. Similarly, the segment polarity gene engrailed is expressed in the posterior compartment of each of the 14 segments and the resulting pattern defects are associated with the posterior part of each segment.
|
|
 |
|
|
|
 |
|
|
|
 |
|
 |
|
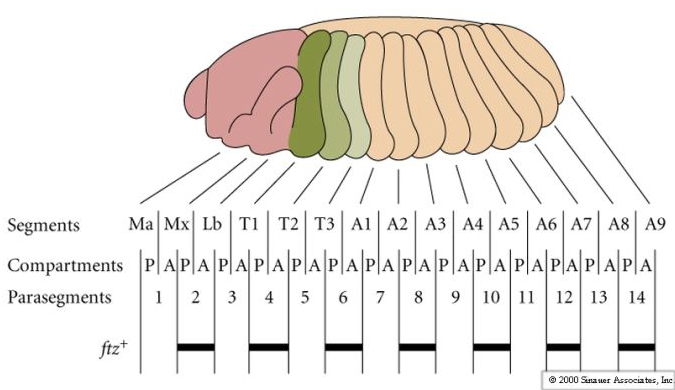
| One unusal finding that surprised fly developmental biologists is that the first indication of segmental periodicity is pair rule gene expression. When first seen it was assumed the seven stripes of gene expression would correspond to segmental borders. However, it turned out that the embryo builds segmental repeat units by first expressing pair rule genes in 'parasegmental" domains. Parasegmental domains straddle the future segmental borders. It is not known why embryos develop segmentation this way, but it certainly complicates teaching! |
|
|
|
 |
|
| The figure at right shows the expression of the major GAP genes. Notice that they divide up the entire anterior posterior length of the embryo into GAP gene expression domains. They are about five more GAP genes not shown so it is clear that there is some overlap. Notice that the cytoplasmic poloarity genes are giving rise to a more complex and finer grain pattern of GAP genes. The way this develops is illustrated below in a simplified diagram of gene regulatory interactions among this network of cytoplasmic polarity and GAP genes. |
|
|
|
|
 |
|
 |
|
| Remember that this pattern of GAP gene expression is occuring in the syncycial blastoderm. The GAP genes are all transcription factors that diffuse freely among the nuclei establishing morphogen gradients (proteins) and domains of gene expression (mRNA). |
|
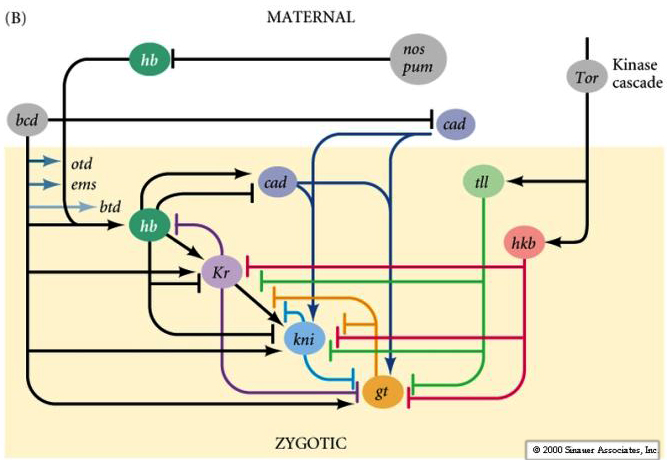
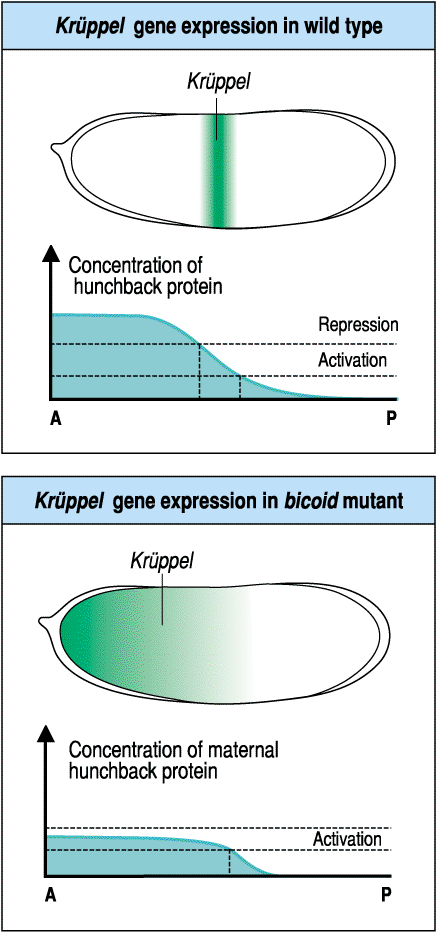
| The maternal gradients of bicoid and nanos effectively antagonize each other to regulate the domains of hunchback and caudal. Notice how the bicoid gradient positively regulates all the GAP genes except Caudal. Then notice the anterior posterior sequence of regulatory interactions among the GAP genes. Hunchback, whose anterior extent is regulated by bicoid, is a positive regulator of Krupple and Caudal, BUT at high concentrations is a negative regulatory of these same two GAP genes. This serves to define and sharpen the border between Hunchback and Caudal. The figure at right shows how at high concentrations huncback is a repressor of kruppel and regulates its anterior border, while at intermediate concentrations hunchback is an activator of kruppel and determines the position and width of the kruppel band. In the bicoid mutant the concentration of maternal hunchback protein is never high enough to repress kruppel. Notice above that Krupple also feedbacks to inhibit Hunchback. In general you often see both positive and negative interactions among neighboring GAP genes. Caudal is a postive regulator of the more posterior GAP genes Knirps and Giant. Notice that the terminal genes tailless and huckabein both inhibit the GAP genes Giant, Knirps, and Krupple. Do you remember what limits the anterior domain of the terminal genes? Groucho. A commom theme during the development of complex patterns is that the information in a morphogen gradient is used to initiate "vague" regions of gene expression, but the subsequent regulatory interactions among the activated genes can lead to more complex and sharply defined patterns. |
|
|
|
|
 |
|
| Next we need to explain how the the GAP genes specify the 7 stripes of Pair Rule gene expression over the region of the embryo that will give rise to the 14 segments. The first thing you should notice about the figure at right that shows the development of ftz expression is that the stripes start "fuzzy" and become sharper with time. Just as with GAP gene regulatory networks, so to the Pair Rule genes feedback on themselves and nearby genes to establish sharp gene expression domains.
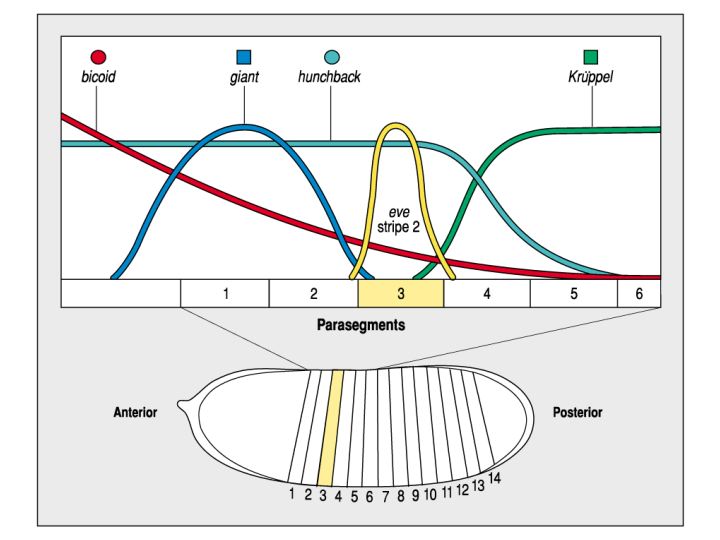
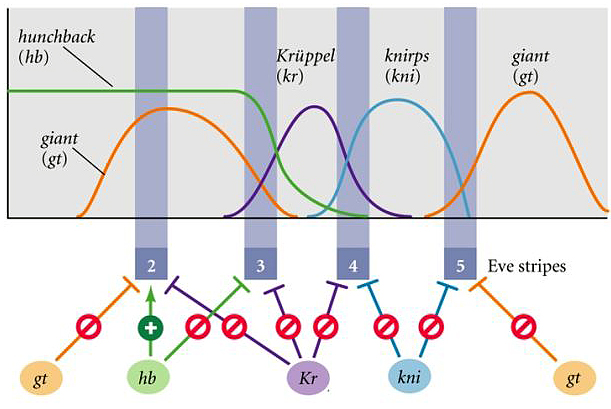
The cytoplasmic polarity and GAP gene morphogen gradients are used to specify the 7 stripes of pair rule gene expression (there are about 8 pair rule genes that are each expressed in 7 stripes across the future 14 segmental domains of the embryo. In the figure below and right you can see how the bicoid, giant, hunchback, and kruppel morphogen gradients can be used to specify even-skipped (eve) stripe 2 in parasegment 3. Just by seeing the location of the eve expression domain and knowing the shape and position of the cytoplasmic polartity and GAP morphogen gradients we can make reasonable predictions about gene interactions responsible for specifying the eve stripe 2.
|
|
 |
|
|
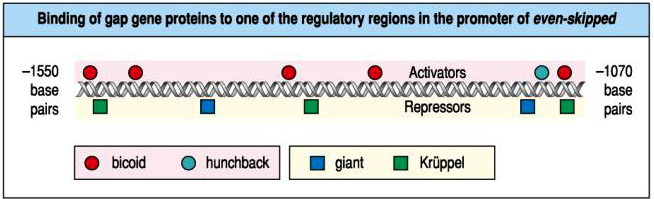
| We would predict that giant is a negative regulator of eve stripe 2 and determines the anterior border. Kruppel is also predicted to be a negative regulatory of eve and determines posterior border of stripe 2. We would predict that either hunchback or bicoid (or both) are positive regulators of eve stripe 2. Experiments to test the role of each of these morphogens in regulating eve stripe two show that our predictions are correct (below). We can demonstrate that giant and kruppel bind to the promoter region and negatively regulate transcription, while bicoid and hunchback bind and positively regulate gene expression. |
|
|
|
 |
|
 |
|
|
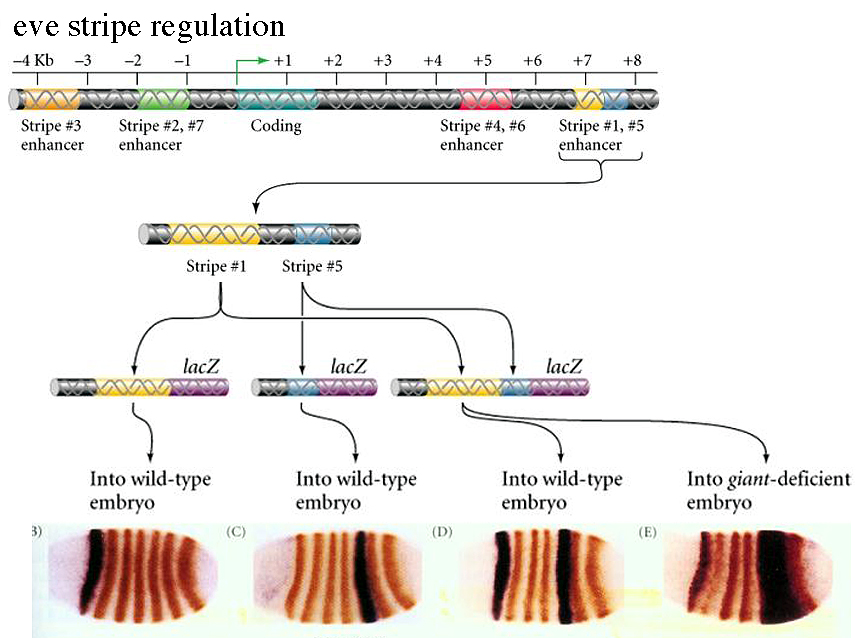
The figure below shows one way that you can experimentally determine the 5' and 3' regulatory regions that control the seven stripes of pair rule gene expression. This is a general techique that can be used to understand the regulation of any gene. |
|
|
| First we must identify the coding region of the gene. This is normally done by directly sequencing the gene. However, we often can't identify all the 5' and 3' untranslated regions that contain enhancer elements important for gene regulation. To identify these we can clone them into a DNA contruct that contains a weak promotor and a "reporter gene". In this case we use lacZ, an enzyme that produces a blue product when given the right substrate. We next inject these "test" constructs into the fly syncycial blastoderm and observe where lac Z is expressed. In the example at right you can see that we have identified the 3' region that regulates stripes 1 and 5. Notice that by injecting into the giant deficient embryo we can show that Giant is a negative regulator of stripe 5 and a positive regulatory of stripe 1. |
 |
|
|
|
 |
|
| The figure at right shows a simplified diagram of how eve stripes 2, 3, 4, and 5 might be regulated by the GAP genes gt, hb, kr, kni, and gt. Notice that for stripes 3, 4, and 5 they don't show any "activators". What would be your hypotheses for postive regulators of these stripes? |
|
|
|
|
|
 |
|
|
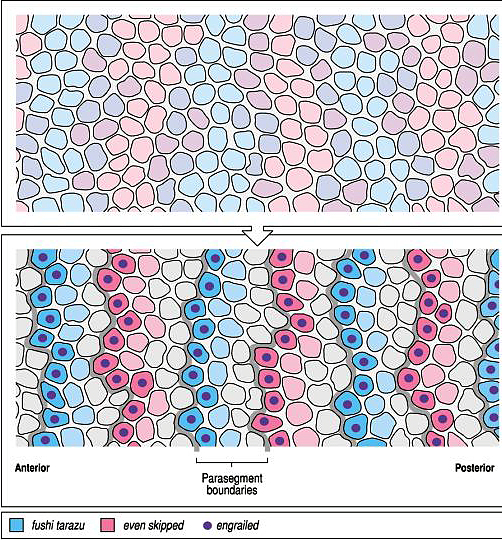
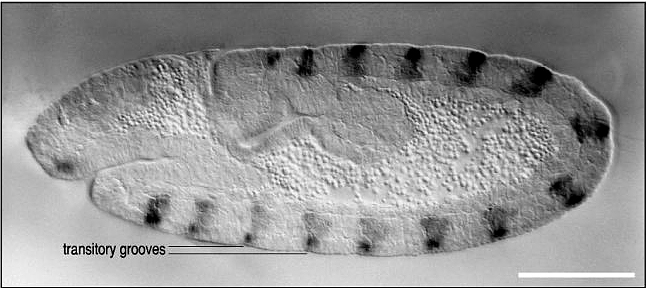
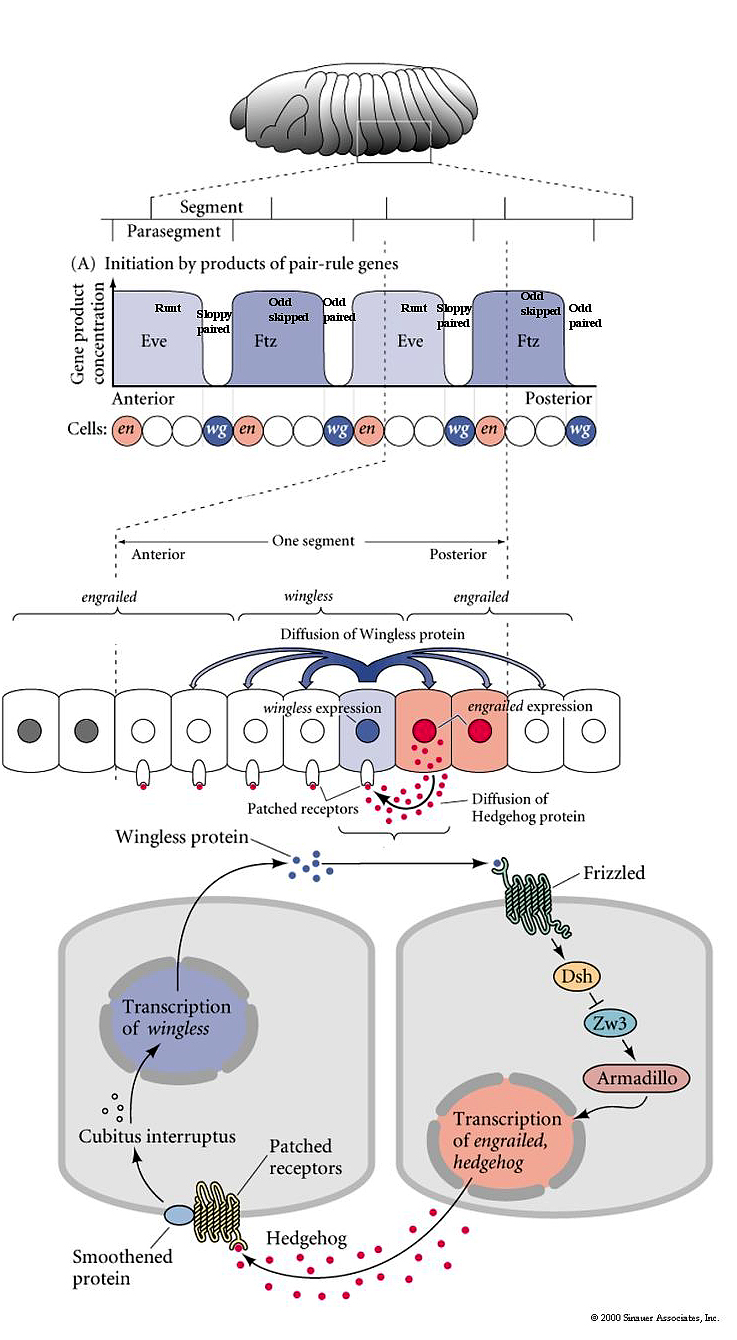
| Remember that pair rule gene expression is established in the syncycial blastoderm. In the cellular blastoderm you can see that the stripes of pair rule gene expression are confined to just a few rows (2-3) of cells in each parasegment. The figure at right shows the expression of the pair rule genes ftz and eve. Remember that there are 8 different pair rule genes expressed across the 14 parasegments. That means that 4 pair rule genes are expressed in each parasegment. Given that there are only 3-4 rows of cells that means that each row of cells in each parasegment will express a unique pattern of pair rule protein concentrations. Remember that all the pair rule genes are also transcription factors. These unique patterns of pair rule protein levels are used to specify the positions of the segment poloarity genes. In the figure at right you can see that the segment polarity gene engrailed is expressed by the single row of cells at the anterior border of each parasegment. Notice that this corresponds to cells expressing the pair rule genes ftz and eve. In the picture at right showing a fly embryo labeled to show engrailed protein expression, you can see the transitory grooves that are associated with the parasegmental borders. Fly biologists first thought these were grooves associated with segment boundaries, but they were fooled. Engrailed is expressed in 14 stripes associated with the anterior border of each parasegment and the posterior region of each future segment. |
|
 |
|
| The segment polarity genes function in a cellular environment requiring a more complex signaling pathway to pass signals between cells. In the figure are right you can see how the segment polarity genes wingless and engrailed establish an organizing center for patterning the across the anterior posterior axis of each segment. The pair rule genes initially activate engrailed expression at the anterior boundary of each parasegment where the concentration of eve and ftz is high. Engrailed is inhibited by high concentrations of odd paired, runt, odd skipped, and sloppy paired. In contrast, wingless is activated at the posterior boundary of each parasegment by high concentrations of the pair rule genes sloppy paired and odd paired , and repressed by eve and ftz. The boundary between wg and eg expressing cells is sharpened and maintained by the signaling interactions between these two rows of cells sit next to each other at the parasegmental border. Engrailed cells secrete hedgehog that diffuses to nearby cells and binds to the patched receptor where it releives the inhibition on the smoothen protein. This leads to the activation of wingless transcription. Wingless protein is secreted and binds to the frizzled protein on engrail positive cells. This leads to the activation of engrailed transcription. This positive feedback loop ensures the maintainence of the adjacent wingless and hedgehog secreting cells. The secreted hedgehog and wingless establish morphogen gradients across each segment that specifies the anterior posterior pattern of structures in each segment. (more on hedgehog signalling pathway) |
|
 |
|
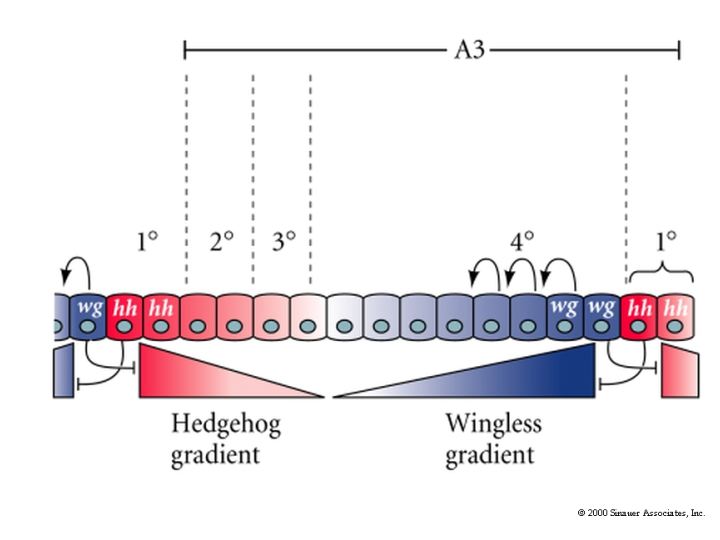
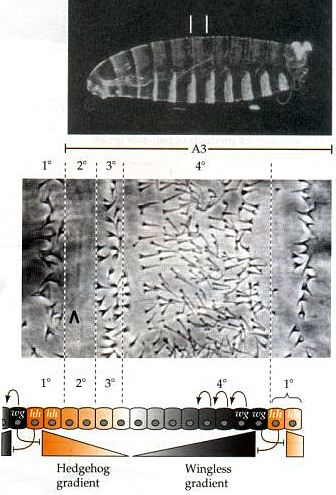
| The hedgehog and wingless gradients are functionally antogonistic. We can observe the effects of the two morphogen gradients by looking at the pattern of denticles along the anterior posterior axis of each segment. We can define morphological zones (1,2,3,4) across each segment and note how threshold concentrations of hedgehog and wingless affect the patterns in each zone. |
 |
|
|
|
This shows a fly larval cuticle prep and then a higer power view of the pattern of cuticular hairs across a single segment. Notice the zones associated with different threshold values of hedgehog and wingless. You can experimentally alter the gradients of hedgehog and wingless and show that the ant-post. pattern zones correspond to threshold values of the two morphogens. Do you see how the hedgehog mutant got its name? In the absence of hedge, the wingless morphogen takes over the entire segment so the anterior-posterior pattern is all like zone 4. A very hairy and hedgehog like segmental pattern across the entire segment. |
 |
|
| We have now created the anterior posterior pattern of a fly with an acron, 14 segments (head, thorax, abdomen) and telson. Amazingly, the fly does this with only about 41 genes of the anterior group (3) , posterior group (7), terminal group (4), GAP (8), pair rule (8), and segment polarity(9). This is so simple in the fly, in part, because the morphogen gradients can be the transcription factors that directly regulate genes in the syncycial blastoderm. Notice that once cells form at the time segment polarity genes are expressed you now need a more involved signaling pathway to regulate the two morphogen gradients of hedgehog and wingless. |
|
|
 |
|
|
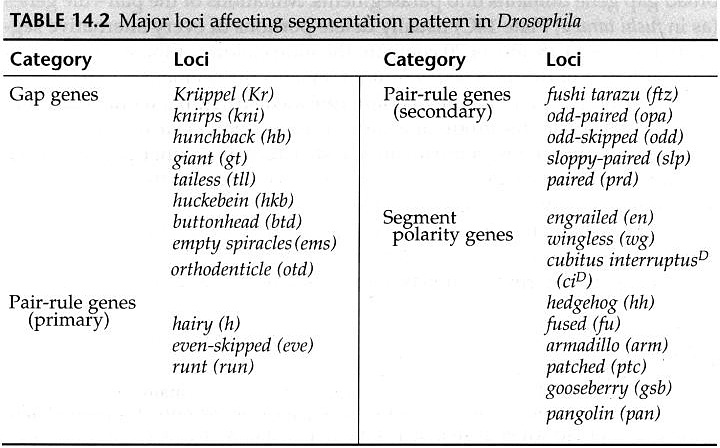 |
|
|
|
 |
|
| Homeotic Selector Genes: HOM complex |
|
| The next step in fly development is to specify the unique identity of each segment. This is done by the homeotic selectory genes of the HOX complex. This is made up of two clusters of genes. The antennnapedia complex and the bithorax complex, both on the fly's third chromosome. The famous fly at right results from a mutation of the ubx gene in the bithorax complex. This mutation causes the third thoracic segment that normally expresses halteres to be transformed into a second thoracic segment that expresses wings! The wings are not functional because essential muscle and nerve components from adjacent segments are not transformed. |
|
| The pictures at right show scanning electron micrographs of a wild type head and a head where the antennae have been transformed into legs (again not function, but otherwise normal). This transformation results from the antennapedia mutation, that causes the antennapedia gene to be misexpressed in the head. These homeotic mutations caused tremendous excitement among developmental and evolutionary biologists because they seemed to be master regulators of segment identity and the obvious substrate for dramatic evolutionary steps. |
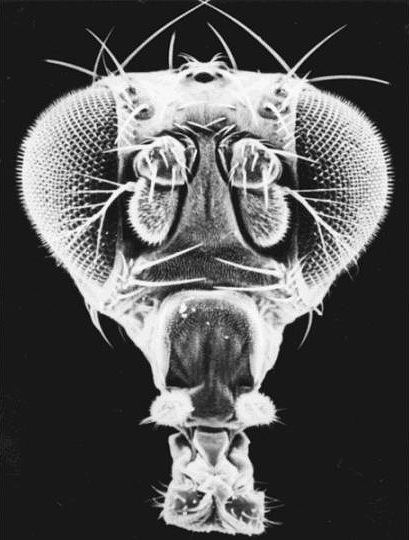 |
 |
|
| The illustration at right shows the genes of the antennapedia complex and the bithorax complex. An amazing finding was that the linear 3' to 5' arrangement of these genes on the third chromosome correlated with the anterior to posterior expression pattern of these genes along the fly embryo. The expression pattern correlated with the specification of segmental identity, but notice that there are only 3 genes of the bithorax complex to specify 9 segments. This is probably accounted for by a combinatorial code, and the fact that the intermediate abdominal segments are very structurally very similar. Generally there is a "posterior dominance" regulatory relationship among genes of the HOM complex. This explains the phenotype of the ubx mutant. When ubx is lost it no longer inhibits the posterior extent of antennapedia. Antennapedia extends into the domain normally held by ubx and transform the T3 segment to a T2 identity. |
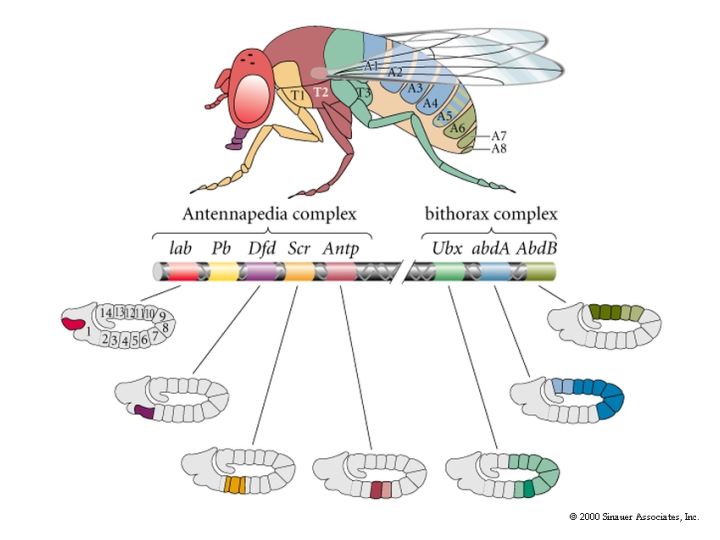 |
|
|
|
 |
|
|
| All the genes of the HOM complex are transcription factors and all contain a highly conserved protein motif call the homeodomain. This is a DNA binding motif of the helix loop helix class. The homeodomain is so highly conserved that human and mouse homeodomains can rescue fly mutants! |
|
|
 |
|
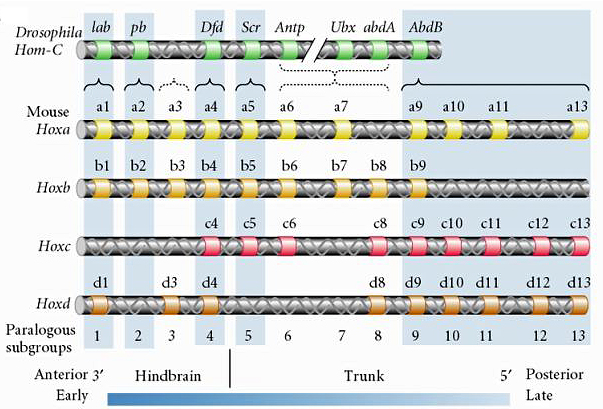
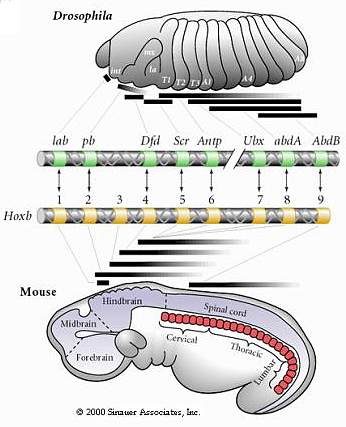
| This leads to the next amazing observation. The fly HOM complex has been conserved in the evolution of vertebrates. In mammals there has been a two fold duplication of the Hom complex to give four paralogous subgroups on four different chromosomes. But amazingly, the order of genes has been conserved. |
|
| Not only has the 3' to 5' order of the genes been conserved, but the pattern of expression of these genes along the anterior-posterior body axis is correlated with the linearly position on the chromosome, just as in fly. Our hypothesis is that the linear position on the chromosome must somehow be involved in the sequential regulation of the genes along the body axis, but we don't have good experimental proof of this because of technical limitations in our ability to manipulate very long regions of DNA. The discovery of the HOX genes was truly the "rosetta stone" for developmental and evolutionary biologists. This finally convinced all scientists that at a very basic level the development mechanisms controling body pattern are highly conserved. The HOX genes are involve in the specification of body pattern in all metazoans. |
|
|
|
 |
|
|
|
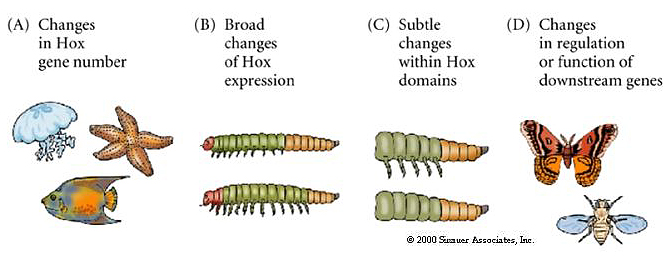
The figure below suggests ways in which the evolution of body complexity can be roughly correlated with the number of Hox genes and the complexity of regulation across phyla and presumable through evolutionary time. |
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|




























